[ad_1]
This study, which investigated the effects of hemodynamic changes and oxygen saturation on cerebral perfusion in infants with CHD, found that the presence of SPS was negatively correlated with perfusion in cGM, but not in dGM. Additionally, infants with CHD had increased cGM cerebral perfusion compared to healthy controls after controlling for SPS, aortic arch occlusion, and oxygen saturation.
Effects of body-lung shunt
The brain-stealing effect caused by SPS is well known and has been assessed by Doppler ultrasound and manifests as a decrease in end-diastolic velocity with an increase in the resistive index.28 and pulsatility index29 Located in the cerebral arteries. To our knowledge, this is the first study to evaluate the association of this effect with regional cerebral perfusion using pCASL MRI data.
We found a strong inverse correlation between relative shunt size and cerebral perfusion in both dGM and cGM. This finding is consistent with a study describing an inverse relationship between aortopulmonary collateral flow and whole-brain perfusion after 0.5 years in single-ventricular patients before the Glenn procedure.30 Using cardiac phase contrast MRI data.
Although we found that dGM perfusion was strongly dependent on relative shunt size, we detected no evidence of a significant effect of the presence of SPS (vs. absence of SPS) on dGM perfusion. In contrast, the presence of SPS significantly reduced cGM perfusion. We conclude that in SPS patients with low diastolic blood flow in cerebral arteries, cGM regions, especially fronto-parietal regions, lack appropriate compensatory mechanisms, favoring perfusion in dGM. The frontoparietal region is known to be the most perfused cortical region in newborns, and frontoparietal perfusion increases further after birth.31,32 The frontoparietal network also supports cognitive functions such as executive function, which are known to be affected in children and adults with CHD.33 Therefore, abnormalities in frontoparietal perfusion during infancy due to the presence of SPS may increase the risk of subsequent neurodevelopmental or cognitive deficits, but are associated with SPS, perioperative perfusion changes, and neurodevelopmental outcomes in CHD infants. Further research may be needed to investigate the relationship between
Although scans indicating the presence of SPS were performed more frequently preoperatively than postoperatively, we found no evidence of a significant interaction between SPS and postmenstrual age.
Effects of aortic arch occlusion and arterial oxygen saturation
Aortic arch occlusion and oxygen saturation were not significantly associated with cerebral perfusion in both dGM and cGM. This lack of significant effect of aortic arch occlusion may have been caused by differences in the degree of aortic arch occlusion, which was not evaluated in detail in this study. Furthermore, the underlying effects of hypoxic responses on cerebral perfusion are very complex, and although cerebral vasodilation has been reported in acute hypoxia, the effects of more chronic hypoxia, especially in CHD patients, are still poorly understood. is not explained.16 In contrast to our findings, Nagaraj et al.11 reported increased perfusion in patients with aortic arch occlusion compared with those without, and lower regional perfusion in cyanotic than in cyanotic CHD. However, they investigated these effects separately and did not adjust for the presence of SPS, which may have resulted in different results. In this study, both aortic arch occlusion and oxygen saturation appear to have less influence on perfusion than the presence of SPS.
Differences between infants with CHD and healthy controls
Although no significant differences in cerebral perfusion were found between CHD patients and controls adjusted for PMA only, adjusting for the presence of SPS, aortic arch occlusion, and oxygen saturation increased gray matter perfusion in CHD patients. found. The complex statistical model had a better fit in predicting cerebral perfusion than the simple model in both gray matter regions. This confirms that hemodynamic and oxygen saturation parameters explain some of the variation in cerebral perfusion in patients with severe CHD and need to be taken into account when investigating brain development. I am.
To date, few studies have evaluated differences in cerebral perfusion between CHD patients and healthy controls. Similar to our findings, previous studies used both pCASL and adjusted only for postmenstrual age to find significant differences in global and regional perfusion between CHD patients and healthy controls. We found no evidence that there was a difference.11 and phase contrasttwenty three MRI sequence. After accounting for SPS, aortic arch occlusion, and oxygen saturation, we found increased gray matter perfusion in infants with CHD compared to controls. This implies compensatory mechanisms to provide optimal cerebral perfusion and oxygen delivery for patients with altered hemodynamic and oxygen saturation parameters. It has also been suggested that the brain-sparing effect may persist even after birth. This brain-sparing effect has been reported in fetuses with severe CHD and consists of a relative reduction in cerebrovascular resistance to placental arterial resistance, increasing cerebral perfusion.Ten
On the other hand, the CHD group also consisted of surgically corrected CHD patients. Gray matter perfusion was increased in CHD patients, independent of the presence of SPS, aortic arch occlusion, and oxygen saturation. This relative hyperperfusion may be explained by overcompensation of cerebral perfusion after normalization of hemodynamic parameters and oxygen saturation, or may be the result of a (temporary) increase in cardiac output, such as due to drug therapy. There may be. Due to limited sample size, the effect of drugs on cerebral perfusion was not investigated in this study. Preoperative administration of prostaglandins is very common in this study population, and diuretics, antihypertensives, analgesics, anticoagulants, and antibiotics are used postoperatively to influence brain autoregulation. There was a possibility. The effects of these drugs on cerebral perfusion in infants with CHD have not yet been investigated in the literature. None of the patients were receiving catecholamines or milrinone (a phosphodiesterase III inhibitor) at the time of the scan.
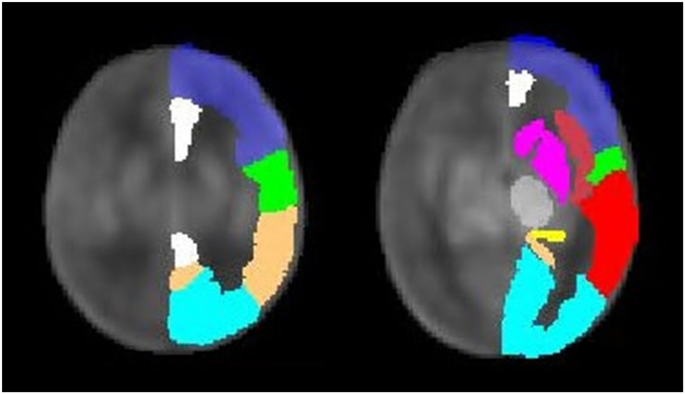
Regional differences in cerebral perfusion
Different regional perfusion patterns were found in gray matter, with cGM perfusion varying depending on hemodynamic influences (particularly the presence of SPS), whereas dGM perfusion was more consistent.
In healthy infants, cerebral perfusion (of the whole brain) increases rapidly with age, which is thought to reflect developing areas such as synapse formation and myelination. At neonatal age, cGM perfusion is lower than dGM perfusion. Within the first few months of life, relative cGM perfusion increases, likely as a result of increased postnatal sensory input. On the other hand, dGM perfusion remains more constant and relative dGM perfusion decreases.34,35,36,37
Therefore, cGM perfusion is likely to be subject to significant changes during the neonatal period and may be more susceptible to hemodynamic effects than dGM. Additionally, gray matter maturation has been shown to be delayed in patients with severe CHD.38 and postpartum39 Volume differences compared to healthy controls were highlighted in cGM.38 Therefore, brain immaturity may also play an additional role in perfusion regulation, as it has already been shown to influence the extent of cerebrovascular autoregulation.40,41 Alternatively, the relationship between brain immaturity and perfusion may be bidirectional, as reduced perfusion is associated with increased brain atrophy over time in adult arterial disease.42 However, within the CHD population, the directionality of the association between cGM maturation, brain volume, and changes in perfusion remains unclear.
Effects of age and gender
Similar to results reported in previous studies, we confirmed that cerebral perfusion in CHD patients increases with age, similar to healthy infants.11 We found no evidence for a difference in this age-dependent perfusion increase in CHD with and without SPS or in patients and healthy controls. Gender was included as a covariate in all statistical models assessing cerebral perfusion for exploratory reasons, given the known sex differences in perfusion that emerge later in childhood and adolescence.43 And it continues even into adulthood.44,45 However, we found no evidence for an effect of gender on cerebral perfusion, and the covariate was subsequently omitted due to limited sample size. No studies were found that comparatively evaluated sex differences in cerebral perfusion in CHD or healthy neonates.
Effects of cerebral perfusion on neural development
The impact of altered cerebral perfusion on neurodevelopmental outcomes in infants with CHD remains to be established.46 Studies of the direct association between cerebral perfusion and neurodevelopmental outcomes in these patients are difficult because the influencing factors change over time, for example due to changes in cardiac structure and function due to surgical or medical treatments. Therefore, measuring cerebral perfusion using MRI reflects current hemodynamics, which can be difficult. Situation only. In this study, we found that patients with CHD had altered cerebral perfusion, whereas patients with CDO had altered cerebral perfusion.2 It was consistent regardless of hemodynamic parameters and between CHD and healthy infants. Therefore, compensatory mechanisms such as hyperperfusion in CHD patients (compared to controls) or increased hemoglobin in SPS patients (compared to patients without SPS) may result in adequate cerebral oxygenation. It remains unclear whether the supply of other nutrients (such as glucose) is adequately supplemented in patients with SPS. A study evaluating preterm patients found that longer duration of open PDA (possible brain steal effect) was associated with decreased cerebellar volume.47 Further studies are needed to investigate the interaction between cerebral perfusion and brain maturation in CHD patients. Our findings suggest that the fronto-parietal region is particularly vulnerable to poor blood supply in patients with SPS and that research focusing on the maturation of this region would be valuable.
Limitations
This study has some limitations. A well-known challenge in CHD research is disease heterogeneity. Using multivariate models, we attempted to overcome this difficulty and gain knowledge about the pathophysiological factors that influence cerebral perfusion. Nevertheless, due to the limited sample size and exploratory study design, it is difficult to investigate the effects of pulmonary-to-systemic shunts on cerebral perfusion and more general influencing factors such as cardiac output, blood pressure, and medications. I couldn’t do that. Arterial oxygen saturation was measured via an MRI-compatible transcutaneous probe, which has been shown to underestimate true oxygen saturation (mean value of 94% in healthy controls). Because oxygen saturation was measured using the same probe in all patients, the effect of this measurement error is thought to be minimal.However, the measurement of oxygen partial pressure (PaO)2) would have been useful.
Although the CHD group showed a wider distribution of age at scan, we found no evidence of significant group differences in gestational age or postnatal age at scan. We also did not find a significant interaction of his PMA and study group in the model.
A predefined AAL mask was applied for regional perfusion assessment of the gray matter. The border areas between cerebrovascular territories are the most vulnerable to hypoperfusion and were not specifically evaluated in this study.
The association between Doppler ultrasound parameters and MRI perfusion variables will be of great clinical interest, as ultrasound is more accessible and suitable as a bedside tool for clinical monitoring of the brain steal effect.28 Unfortunately, due to the different time points of examination (median 5, IQR 4-7 days between ultrasound and MRI), this study does not adequately correlate routinely generated Doppler ultrasound and pCASL perfusion data. could not be analyzed. Cerebral perfusion between these time points can vary based on time-dependent changes in oxygen saturation and drugs, as well as age.
[ad_2]
Source link






