[ad_1]
March 15, 2024 — BioCardia, Inc., a biotechnology company focused on advancing late-stage cell therapy interventions for cardiovascular disease; Technology company StemCardia, Inc. today announced a long-term partnership to advance StemCardia’s investigational pluripotent stem cell product candidate for the treatment of heart failure.
Under this collaboration, BioCardia will become the exclusive biotherapy delivery partner for StemCardia’s cell therapy candidates, leading to FDA approval of an Investigational New Drug Application (IND) and subsequent anticipated Phase I/II clinical development. This will be done through promising research.
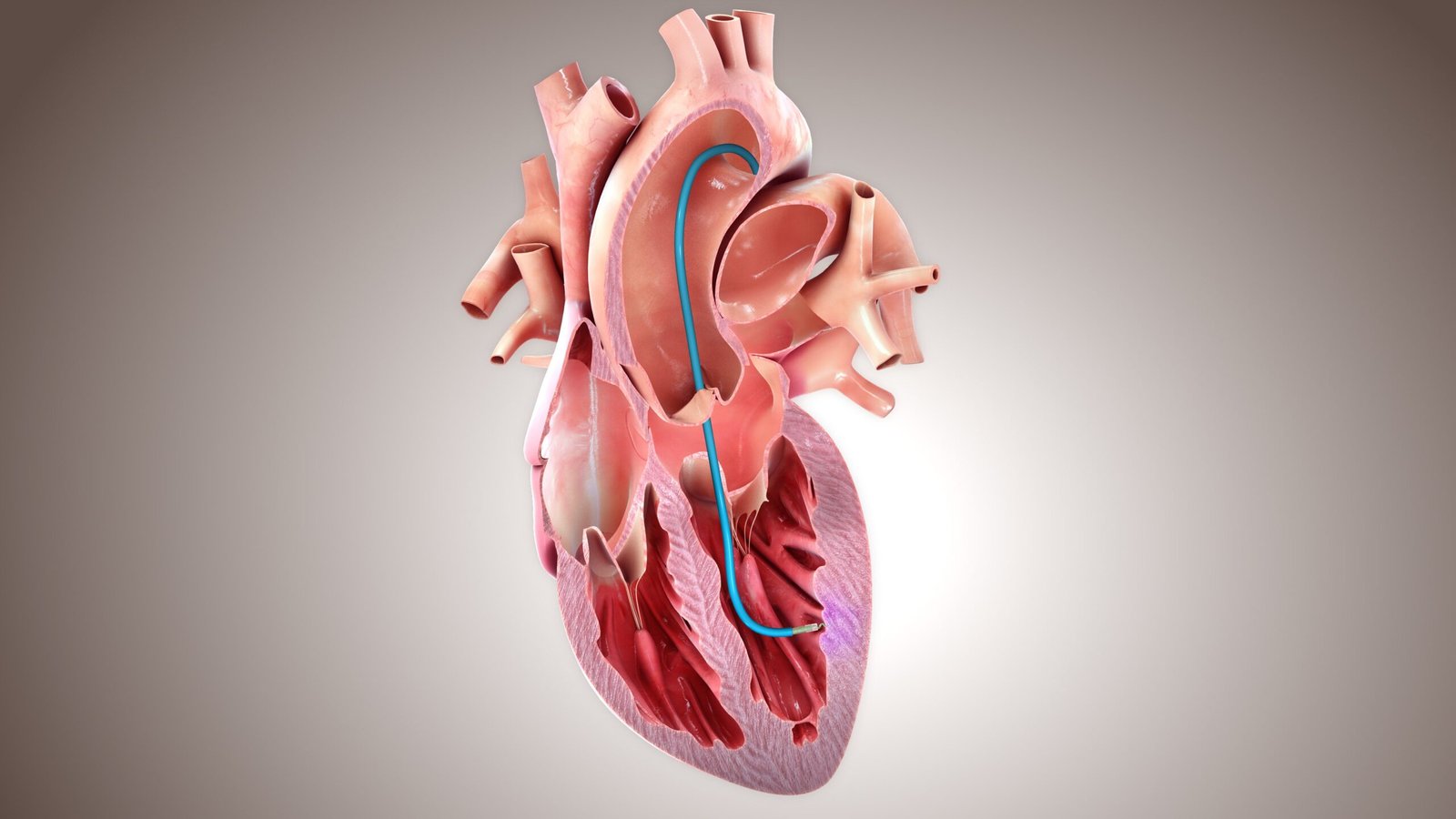
“BioCardia has established a way to safely deliver cellular medicines directly to the heart,” said Chuck Murry, MD, PhD, Founder and CEO of StemCardia. “We have successfully collaborated with BioCardia to introduce our authentic cardiomyocytes into large animal models of heart failure, and this collaboration will accelerate clinical development and provide future commercial access to off-the-shelf cardiac regenerative therapies. We’re excited to expand.”
“StemCardia’s team includes recognized leaders in the field of cardiac regenerative medicine who pursue sophisticated strategies to repair failing hearts. Our experienced team and track record We look forward to supporting their efforts with our unique Helix biopharmaceutical delivery system,” said Dr. Peter Altman, CEO of BioCardia. “This partnership will enhance future treatment options for millions of people suffering from heart failure, offset the biopharmaceutical delivery development costs of our proprietary programs, and ensure that our efforts contribute to StemCardia’s therapeutic development success.” Hopefully, we will provide meaningful revenue distribution to our investors.”
For more information, please visit www.biocardia.com.
[ad_2]
Source link






