[ad_1]
Study design and participants
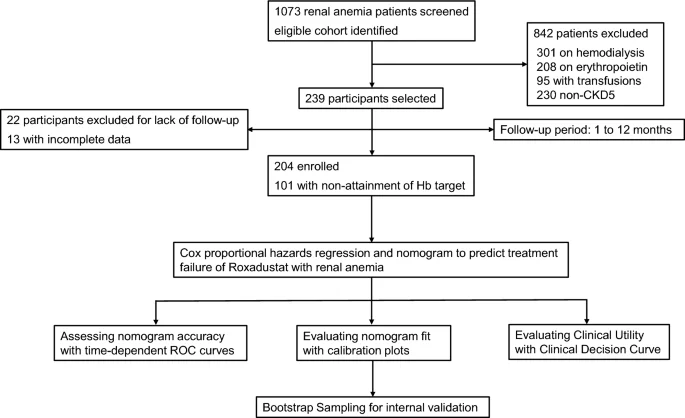
This single-center retrospective study focused on patients with PD and renal anemia treated with roxadustat from January 1, 2019 to January 31, 2023 at the Department of Nephrology, Nanjing Drumlou Hospital. analyzed the data. The study excluded patients receiving only hemodialysis, blood transfusions, ESAs, or iron administration, and patients with incomplete clinical data or insufficient his 1-year follow-up. Inclusion criteria included patients with stage 5 CKD and renal anemia undergoing PD and being treated with roxadustat. This study was approved by the Medical Ethics Committee of Nanjing Drumlou Hospital (ethics approval number: 2023-118-01), written informed consent was obtained from all participants before data collection, and all procedures were performed in accordance with the Declaration of Helsinki. We followed the principles. .
therapeutic intervention
Roxadustat capsules are orally administered three times a week with specifications of 20 mg/50 mg per tablet (Domestic drug approval number H20180024), with doses of 50 mg, 70 mg, and 100 mg based on patient follow-up. , adjusted from 120 mg. Increase Hb level. Initial doses were determined by body weight, with patients weighing <60 kg receiving 100 mg per dose and patients weighing ≥60 kg receiving 120 mg per dose. Patients transitioning from ESA to roxadustat therapy will receive 70 mg per dose if rHuEPO is less than 4500 U/week and 100 mg per dose if rHuEPO is 4500 U/week or higher. I did. Physicians adjusted the dose by considering the patient's Hb level at follow-up and personal factors.
Data collection
Basic demographic and clinical data, including 1-year follow-up Hb levels, were collected from the health information system (HIS) and telephone interviews with participating patients. Demographic profile included age, gender, body mass index (BMI), and smoking status. Clinical data included PD duration, initial dose of roxadustat, primary renal disease, diabetes, hypertension, cardiovascular disease (coronary artery disease, cerebrovascular disease, heart failure, cardiomyopathy, arrhythmia, valvular heart disease, myocardial disease). comorbidities such as inflammation (including inflammation) were included. peripheral artery disease), infections, liver cirrhosis, gastrointestinal bleeding, etc. Additional data included concomitant medications (iron, phosphorus binders, statins) and reticulocyte count (Ret), Hb, erythropoietin (EPO), transferrin saturation (TSAT), serum ferritin (SF), serum transferrin (sTf), Total carbon dioxide (TCO2), platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), serum phosphate, brain natriuretic peptide (BNP), alkaline phosphatase (ALP), albumin (ALB) , serum creatinine (Scr), C-reactive protein (CRP), estimated glomerular filtration rate (eGFR), total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL). Follow-up data collection ended on September 1, 2023.
Clinical efficacy evaluation and group
This study evaluated the clinical efficacy of roxadustat in treating anemia in patients in line with the “Chinese Clinical Practice Guidelines for Diagnosis and Treatment of Renal Anemia”.7 and “Chinese expert consensus on the treatment of renal anemia”12. Evaluation was based on her Hb levels throughout the 1-year follow-up period. A Cox proportional hazards regression model was employed using time-related events as outcome measures. The outcome variable was defined as failure to achieve target Hb level at 1-year follow-up. The time variable was expressed as the period from the start of roxadustat treatment to the time point when the target Hb level was not reached. Those with follow-up Hb < 110 g/L were included in the nonattained Hb target level group, and the others were included in the achieved Hb target level group.
statistical analysis
Data cleaning and preparation was performed in SPSS 26.0, and all hypothesized predictor variables were encoded as categorical variables and quantified using case counts and percentiles. Development, visualization, and validation of the Cox proportional hazards regression model was performed using R 4.2.3 software. Sample size estimation was performed based on the 10 EPV (10 EPV) method, and cases with missing data were systematically excluded. Data import was facilitated by the “foreign” package in R. Univariate and multivariate analyzes and fitting of Cox proportional hazards regression models were performed using the “survival” package and the “coxph” function.variable with pValues less than 0.2 in univariate analysis were considered for inclusion in the multivariate Cox proportional hazards regression model. Predictor variable selection was performed through stepwise regression analysis based on AIC (Akaike Information Criterion), favoring the model showing the lowest AIC value. The final model included the following variables: p– A value greater than 0.05 if it contributes to the minimum AIC. The “rms” package was used to develop a nomogram to predict the probability of treatment failure with roxadustat. We used the ‘survivalROC’ package and the ‘predict’ function to estimate predicted probabilities at 6 and 12 months post-treatment. Model discrimination was assessed by fitting survival ROC curves at these time points using the Kaplan-Meier (KM) method. A higher area under the curve (AUC) value indicates better discrimination. To assess the accuracy of the model, we generated calibration plots using the “calibrate” function of the “rms” package. Clinical utility was assessed by decision curve analysis (DCA) performed using the “stdca” package. The ‘plot’ function makes it easy to visualize these curves. Internal validation was performed using a bootstrap self-sampling method with 1,000 resampling iterations using the “boot” function.
Ethics approval and consent
This study was approved by the Medical Ethics Committee of Nanjing Drumlou Hospital (ethics approval number: 2023-118-01). Written informed consent was obtained from each participant before data collection, and all procedures were performed in strict accordance with the principles of the Declaration of Helsinki.
[ad_2]
Source link






