
[ad_1]
Introduction
Coronary artery disease (CAD), a major contributor to the overall burden of death in the human population, related to pathological process of atherosclerosis, is mainly elucidated as a series of heart disease caused by myocardial ischemia, hypoxia, or necrosis.1 The formation and advancement of atherosclerosis is closely connected to inflammation. Many previous contributions have shown that the inflammation is closely bound with the development of coronary atherosclerosis, and is the key characteristic of atherosclerosis and its clinical signs. Therefore, it is necessary to monitor inflammatory markers. However, new biomarkers of inflammation are needed to boost the diagnostic precision and provide prognostic insights into atherosclerosis.
White blood cell (WBC) count is a widely used inflammatory marker in the clinical practice, which has been demonstrated to be an autonomous risk factor of MI, unstable angina, and stable CAD with a history of MI.2,3 As we know, white blood cells include lymphocytes, neutrophils, monocytes and eosinophils. The study of Zhao et al4 investigated the value of total and differential leukocyte counts in assessing long-term prognosis of patients with three-vessel disease (TVD), and showed that total WBC count was an independent prognostic element of death and major adverse cardiovascular and cerebrovascular events (MACCEs). Furtherly, lymphocyte-, monocyte-, and eosinophil counts were independent predictive factors of death. Also, monocyte- and eosinophil counts were independent predictors of MACCEs. In addition, the association between novel inflammation-related laboratory parameters and the prognostic value in patients with CAD has garnered widespread interest among researchers. For instance, Hayıroğlu Mİ’s team discovered that a newly defined index – The systemic immune-inflammation index (SII) – is an independent predictor of long-term mortality and intracardiac defibrillators (ICDs) therapy in patients with HFrEF.5 The Naples score (NS), which combines cardiovascular risk factors like neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, albumin, and total cholesterol, is a prognostic tool for cancer patients. The team above also found that NS is independently linked to long-term mortality in ST-segment elevation MI (STEMI) patients receiving PCI.6 Importantly, Zhao et al found that the addition of monocyte-, lymphocyte-, and eosinophil counts increased the prognostic value of the SYNTAX II score for death.
Prognosis of patients with ACS undergoing PCI is always a key point of the clinical attention. Most prognostic studies have focused on total WBC, neutrophils, monocytes, and lymphocytes; however, few researches pay attention to the predictive value of eosinophils in ACS patients. We aimed to investigate the association between the absolute eosinophil count (AEC) on admission and MACCEs in ACS patients who receiving PCI, and to determine whether the addition of AEC could improve prognostic models based on the SYNTAX II score. We hope the results may benefit clinicians on assessments and treatments for patients.
Methods
Design of Study Methods
This study represented a retrospective analysis affiliated to a prospective observational study. Its primary objective was to ascertain the prognostic significance of novel risk factors and the improved Logistic Clinical SYNTAX Score for major adverse cardiovascular events in patients with ACS receiving PCI, from June 2016 to November 2017, who were enrolled at our CV center. ACS, categorized into unstable angina (UA), non-ST-segment elevation MI (NSTEMI), and ST-segment elevation MI (STEMI), was diagnosed based on current guidelines. According to the exclusion criteria, patients with history of malignant tumor, history of rheumatic disease, prior coronary artery bypass grafting (CABG), infectious disease, and miss of follow-up data (four persons in total) are excluded in our study. The final analysis was conducted on 1711 patients in total. Details can be found in the flow chart of patients (Figure 1). This study was carried out according to the Declaration of Helsinki and obtained approval of the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (IRB number: 2016034x). In view of the retrospective feature of this study, we waived the informed consent.
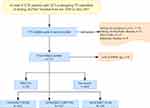 |
Figure 1 Flow chart. Abbreviations: ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; CABG, Coronary Artery Bypass Grafting; MACCEs, major adverse cardiovascular and cerebrovascular events; AEC, absolute eosinophil count. |
Endpoints and Follow-Up
The primary endpoint was MACCEs, which was defined as a composite of cardiovascular death, non-fatal MI, and non-fatal stroke. All outcome data have been meticulously collected since the procedure of angiography. The in-hospital information was acquired by reviewing medical records, and long-term clinical outcomes were obtained through surveys with telephone follow-up or medical visits. All unfavorable events, after carefully checked and confirmed, were recorded by a group of specific clinical physicians. The training of investigators, use of blinded questionnaires, and telephone recording were implemented to ensure the quality of data.
Patient Data
As soon as patients were admitted, demographic data, medical and medication history, and physical examination were recorded using a standard questionnaire. Cardiovascular risk factors, such as age, sex, body mass index, smoking habits, hyperlipidemia, arterial hypertension, diabetes mellitus, history of MI, history of coronary revascularization, and history of cerebrovascular events, were recorded in detail. The data were checked on the day the patient was discharged.
Laboratory Parameters and Measurements
Once patients were admitted after receiving PCI, a complete set of routine laboratory investigations were performed at the central laboratory of Beijing Anzhen Hospital, including whole blood cell test, high-sensitivity C-reactive protein (hs-CRP), global coagulation tests, glycosylated hemoglobin A1c, serum creatinine, plasma triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and total cholesterol (TC). Blood samples were post-operative, obtained in all patients upon back to the unit, and were analyzed no more than 1 hour. Differential WBC counts are calculated in G/L (109/L). Reference values in our laboratory are 3.5 to 9.5 G/L for leukocytes, 1.8 to 6.3 G/L for neutrophils, 1.1 to 3.2 G/L for lymphocytes, 0.1 to 0.6 G/L for monocytes, 0.02 to 0.52 G/L for eosinophils, and 0.0 to 0.06 G/L for basophils. AEC was calculated as absolute eosinophil count (×109/L). Calculation formula for body mass index (BMI) was body weight in kilograms divided by the square of body height in meters (kg/m2). Patients were diagnosed as hypertension when the blood pressure ≥140/90 mmHg and/or receiving anti-hypertensive medications. Diabetes was diagnosed as fasting plasma glucose (FPG) ≥ 7.0 mmol/L, 2-h plasma glucose of 75-g oral glucose tolerance test ≥11.1 mmol/l, previous diagnosis of diabetes and/or chronic hypoglycemic drug-usage. Patients with triglycerides > 1.69 mmol/l, LDL-C > 3.36 mmol/l, HDL-C < 1.03 mmol/l, total cholesterol > 5.17 mmol/l and/or history use of hypolipidemic drugs were defined as dyslipidemia. Patients with a prior history of ischemic stroke or transient ischemic attack were considered to have cerebrovascular accident (CVA). Cardiac failure was diagnosed in patients with symptoms related to congestive heart failure (CHF), or objective indicators of reduced ejection fraction (LVEF < 40%). Criteria for peripheral artery disease (PAD) was vascular diseases related to the aorta and arteries other than coronaries, with the company of exercise-related intermittent claudication, reduced or absent pulsation, angiographic stenosis of more than 50%, revascularization surgery or combinations of all these factors. The SYNTAX (Synergy between PCI with TAXUS and Cardiac Surgery) score was worked out using a calculator (https://syntaxscore.org) for each subject by a group of researchers blinded to the clinical data.
Statistical Analyses
The subjects were categorized into three groups according to tertiles of AEC on admission (T1: ≤ 0.08; T2: 0.08–0.14; T3:>0.14). Continuous variables were presented as the mean ± standard deviation or the median and interquartile range (IQR) when consistent with a normal or non-normal distribution. Categorical variables were expressed as numbers and percentages. The t-test or Mann–Whitney U-test was used properly for continuous variables between two groups. To analyse differences among three groups, the ANOVA or the Kruskal–Wallis H-test was applied to continuous variables. The Pearson Chi-squared test (χ2 test) or Fisher’s exact test was used for categorical variables. Kaplan–Meier methods were implemented to obtain the cumulative events at follow-up and to plot time-to-event curves stratified by AEC tertiles, and then the Log rank test was used to compare differences among groups. Also, Cox proportional hazards regression analysis was performed to estimate the hazard ratios (HRs) and their 95% confidence intervals (CIs) for occurrence of the primary endpoint. The AEC was analyzed in two ways: (1) as a continuous variable; (2) as a categorical variable. Factors with clinical importance and statistical significance in univariate analysis were included in the multivariate analysis. Finally, 13 variables were included in the final multivariate regression model, including AEC, age, sex, hypertension, smoking status, diabetes, cardiac failure, prior PCI, previous MI, peripheral artery disease, SYNTAX I score, hs-CRP, and complete revascularization. We conducted a likelihood ratio test to explore interaction and tested the proportional hazard assumption by demonstrating no importance of variables multiplied by time as time-dependent variables. To visually demonstrate the association between the AEC index and the occurrence of the primary endpoint event before and after adjusting for multiple factors, we utilized GraphPad Prism (version Prism 8.0.2) to create histograms showing the HR and 95% confidence intervals for the three groups respectively, with the first group serving as the control. Finally, we drawn receiver operating characteristic (ROC) plots to determine cut-off values of AEC for predicting the occurrence of the primary endpoint. Area under the curve (AUC) analysis was used to evaluate the prediction of MACCEs. The DeLong test was used to evaluate the prediction of the primary endpoint with the SYNTAX II score alone or combined with the AEC.
A P value < 0.05 was deemed to be statistically meaningful. Statistical analysis was carried out using SPSS version 25.0 software (IBM Corporation, Chicago, IL), and R software (version 4.2.2, R Foundation for Statistical Computing, Beijing, China).
Results
Of the 1711 patients included, the mean age was 60 ± 10 years, and 76.8% of patients were male (n = 1314). The baseline clinical and laboratory characteristics of the study patients according to the AEC tertiles are presented in Table 1. Higher AEC tertiles were associated with higher levels of BMI, hs-CRP, leukocyte, glycosylated hemoglobin A1c, FPG, creatinine and triglycerides, but lower HDL-C levels. The proportion of male patients and current smokers prominently increased with the increase in the AEC tertiles. In terms of comorbidities, the study analyzed factors such as family history of CAD, hypertension, DM, dyslipidaemia, past PCI, previous MI, previous CVA, PAD, and cardiac failure. Through statistical analysis, it was found that patients with dyslipidaemia and those with previous MI showed a significant increase with the elevation of AEC tertiles (Table 1). In terms of ACS subtypes, we did not find any difference (Table 1). Discharge medications, angiographic findings and procedural results of the study patients according to the AEC tertiles are listed in Table 2. Information about antiplatelet therapy can be found in Table 2. Since only 8 people in the whole subjects were treated with rivaroxaban and anticoagulant was not statistically significant, anticoagulant therapy were not list. Medications at discharge across the three groups were similar. Patients with higher AEC tertiles had higher SYNTAX I score.
 |
Table 1 Baseline Clinical and Laboratory Characteristics of the Study Patients According to the AEC Tertiles |
 |
Table 2 Discharge Medications, Angiographic Findings and Procedural Results of the Study Patients According to the AEC Index Tertiles |
The median follow-up duration was 31 months (IQR, 31–37 months). During the follow-up period, there were 44 cases of all-cause death (37 cardiovascular death), 24 non-fatal strokes and 49 non-fatal MIs. Accordingly, 101 patients (5.9%) underwent at least one primary endpoint event. Compared with patients in T1, patients in the higher tertiles had significantly higher incidence of MACCEs (8.5% vs 4.7% vs 4.7% in T3, T2 and T1 group respectively, P = 0.008) (Table 3). There was a significant difference in non-fatal stroke (2.7% vs 1.7% vs 0.3, p = 0.008); however, there were no differences in all-cause death, cardiovascular death, and non-fatal MI.
 |
Table 3 Adverse Cardiovascular Events According to AEC Tertiles During Follow-Up |
Kaplan–Meier survival analysis was shown in Figure 2. The cumulative incidence of the primary endpoint exhibited an escalation with escalating AEC tertiles (Log rank test, P = 0.008). The discrepancy observed in the cumulative incidence of the MACCEs was primarily propelled by an escalation in non-fatal stroke incidents (Log rank test, P = 0.008).
 |
Figure 2 The AEC index and risk: Kaplan–Meier curves for the incidences of the primary endpoint (a), cardiovascular death (b), non-fatal stroke (c), non-fatal myocardial infarction (d) among the 3 study groups based on the AEC index tertiles. |
After adjusting for confounding factors by using multivariate Cox analysis, AEC was an independent risk factor of MACCEs, either as a categorical or a continuous variable. Compared with those in the lowest AEC tertile (T1), patients in the higher tertiles had incrementally higher risk of MACCEs (T2: HR 0.995, 95% CI: 0.582–1.703; T3: HR 1.848 95% CI: 1.157–2.952; P for trend = 0.008). (Table 4, Figure 3). Also, when considering AEC as a continuous variable, AEC was significantly associated with an increased risk of MACCEs (HR of 11.555, 95% CI: 3.318–40.239, P < 0.001). (Supplementary Table 1).
 |
Table 4 Relationship Between the Incidence of the Primary Endpoint and the AEC Index Expressed as a Categorical Variable |
 |
Figure 3 Risk of cardiovascular death, non-fatal stroke or non-fatal myocardial infarction, according to tertiles of the AEC index. Error bars indicate 95% confidence intervals. The first tertile is the reference. (a) Univariate relationship. (b) Relationship adjusted for age, sex, SYNTAX II score, hs-CRP, never smoking, hypertension, diabetes, presence of cardiac failure, PAD, past PCI, previous MI and complete revascularization. |
In addition, considering the potential impacts of comorbidities, medications and ACS subtypes, we further analyzed the association between these factors and the occurrence of MACCEs (Table 5). Table 5 demonstrated that Aspirin was a protective factor for MACCEs, while past PCI, previous MI, PAD, cardiac failure, ACEI/ARB, and NSTEMI were risk factors for MACCEs. Considering the impact of surgical procedures on clinical outcomes, we further analyzed the relationship between PCI access routes and MACCEs events. In this study, the majority of patients underwent PCI through the radial artery route, with a total of 1684 cases and a MACCEs event rate of 5.8% (98/1684). The femoral artery route was used in 25 cases, with a MACCEs event rate of 8.0% (2/25). Two patients underwent PCI through both the radial and femoral artery routes, with a MACCEs event rate of 50.0% (1/2). (P = 0.645, Supplementary Table 2).
 |
Table 5 Relationship Between the Incidence of the Primary Endpoint and Comorbidities, Medicines and ACS Subtypes |
To assess whether the combination of AEC with the SYNTAX II score could improve the predictive value, we evaluated the model performance after the addition of AEC to the SYNTAX II score. After combining the AEC with the SYNTAX II score, the AUC was improved from 0.701 (95% CI:0.646–0.756) to 0.728 (95% CI:0.677–0.780) (DeLong’s test, P = 0.020). (Figure 4).
 |
Figure 4 ROC analysis showing the cut-off values of AEC (A), SYNTAX II score (B) and the adjustment of SYNTAX II Score by AEC (C) to predict the primary endpoint. The primary endpoint was defined as a composite of cardiovascular death, no-fatal MI or stroke, and the individual components of them. Abbreviations: ROC, receiver operating curve; AEC, absolute eosinophil count; AUC, area under the curve; MI, myocardial infarction. |
Discussion
In the present study, we demonstrated that high AEC can be an independent risk factor for MACCEs in patients with ACS undergoing PCI. Moreover, the inclusion of AEC enhanced the predictive utility of the SYNTAX II score for MACCEs.
Eosinophils are generally thought to play a vital role primarily in allergic reactions and host defense against parasites, indicating allergic inflammation, but in recent studies, eosinophils have been shown to be closely related to cardiovascular diseases,7,8 such as coronary thrombosis9 and coronary artery spasm.10 Of note, eosinophils were demonstrated to be related to stroke and found to be more numerous in patients with stroke compared to those with MI.11 In the study conducted by Novotny J. et al, the composition of thrombi, especially the varying abundances of leukocyte subsets, differed markedly between patients with acute ischemic stroke and those with MI. Additionally, the presence and quantity of neutrophil extracellular traps were strongly linked to the clinical outcomes of the patients, suggesting a significant influence of these traps on thrombus dynamics in both conditions.11 This may provide some histological basis for the results of this study.
In the perspective of relevance to emerging cardiovascular diseases (CVD), it has been reported that individuals with high eosinophils at baseline are more likely to develop CVD during follow-up than those with low eosinophils.12 The absolute eosinophil count (AEC) increased after acute MI.13 Sequence variants affecting eosinophil numbers were also associated with risk of MI.14
In terms of clinical prognosis, AEC may be an accurate and independent predictor of CAD severity15 and adverse cardiovascular events. The study of Zhao et al showed that increased eosinophils were associated with poor long-term prognosis of coronary heart disease.4 Toor et al reported that higher eosinophil count initially triggered reduced mortality after PCI, but things overturn after 6 months.3 Although studies have proposed eosinophil count and eosinophil to white blood cell ratio as refreshing biomarkers for risk stratification in patients with coronary artery disease,16 the specific association of eosinophils with atherosclerosis and adverse cardiovascular events is not fully understood. From our data analysis, increased AEC was related to increased incidence of MACCEs among patients with ACS undergoing PCI.
Comorbidities, medications and ACS subtypes may have potential impacts on MACCEs and protective or risk factors can be found in Table 5. However, upon examining the Tables 1–2, we observed that only previous MI showed significant differences among the three AEC tertiles. Therefore, despite the potential impact of these factors on the outcome events, our multi-factor adjusted Cox analysis had largely minimized their biasing effects on the results. Furthermore, the surgical access route (radial artery/femoral artery) and the skill of doctors may affect clinical outcomes. A study conducted by Zabojszcz M et al examined the relationship between the surgical experience of doctors and the mortality rate of patients receiving PCI treatment.17 The study found that when doctors performed a higher volume of surgeries, the perioperative mortality rate of patients was lower. Our center, being a top-tier chest pain diagnosis and treatment center in China with surgical volumes ranking at the forefront, conducted this study on patients operated by equally experienced doctors, minimizing the influence of experience on surgical outcomes. Both radial artery and femoral artery approaches were used, with the majority of patients undergoing PCI through the radial artery route. However, there was no difference between the groups (Supplementary Table 2). In addition, standardized diagnosis and treatment procedures are followed for all patients, thus limiting the impact of interventional time, complications and recovery post-PCI on the primary endpoint in ACS patients, apart from individual variations.
One theory is that eosinophils can store and release tissue factors and proteins that generate reactive oxygen species, including eosinophil cationic protein (ECP), cationic proteins major basic protein (MBP), eosinophil peroxidase (EPX) and eosinophil neurotoxin, which cause endothelial injury, inflammation and platelet activation.18,19 Eosinophil degranulation products are of cytotoxicity and tend to damage and activate endothelial cells.20 Eosinophils are involved in acute coronary events by releasing eosinophilic mediators that trigger atherosclerosis and promote coronary plaque progression and rupture.21,22
Virmani et al published a report of a patient who developed local hypersensitivity to a sirolimus-eluting stent, suggesting that hypersensitivity resulted in thrombosis.23 Some studies investigated the inflammatory response and inflammatory cell infiltration after stent implantation, suggesting that allergy-mediated inflammation may be related to drug-eluting stent (DES) restenosis and thrombosis formation.24–26 Giampaolo Niccoli1 et al further demonstrated that enhanced baseline eosinophil activation was a predictor of MACCEs after DES stenting.27 Our study showed that the higher the baseline AEC level, the higher the proportion of patients found with restenotic lesions which suggested the association between eosinophils mediated inflammation and restenosis did exist.
The SYNTAX I score reflects the severity and complexity of coronary artery disease, provides risk stratification for patients and benefits preliminary choices of surgical methods28,29 according to clinical guidelines.30 Previous studies have shown the SYNTAX I score is an independent predictor of MACCEs in patients undergoing PCI. The SYNTAX II score adds clinical variables on top of the SYNTAX I score, allowing for a more comprehensive and personalized assessment of patients.31,32 Therefore, the SYNTAX II score has a broader clinical application scope. For example, research has found that it is a simple and feasible rapid risk stratification tool for patients with STEMI complicated by cardiogenic shock undergoing PPCI treatment, demonstrating its initial clinical value.33 Of course, more research is needed to further explore and enhance its clinical predictive ability. Since eosinophils were an independent predictor of MACCEs in our study, we combined eosinophils with the SYNTAX II score and our study proved this combination could optimize the predictive value for MACCEs in ACS patients undergoing PCI. The new model may further help clinical decision making.
In addition, we also focused on other relevant researches on coronary heart disease prediction models. The Intermountain Risk Score (IMRS), is composed of inexpensive and widespread laboratory variables and is obtained using a simple formula.34 We found that the IMRS has been regarded as an important laboratory parameter for patients with coronary artery disease. Çınar T et al had explored the IMRS. The team confirmed that the IMRS can predict short- and long-term prognosis of patients with STEMI. Further, the IMRS’ predictive value for overall mortality was non-inferior compared with Thrombolysis in Myocardial Infarction (TIMI) and Global Registry of Acute Coronary Events (GRACE) scores.35 Also, IMRS can predict both short- and long-term mortality in individuals with STEMI accompanied by cardiogenic shock.36 In our article, we demonstrated the predictive role of AEC in the prognosis of ACS patients receiving PCI treatment, and improved the predictive performance of the SYNTAX II score by incorporating AEC. Given this, we also speculate that the inclusion of AEC may further enhance the predictive performance of other scoring systems.
Limitations
Our study has several important limitations. First, this is an observational study conducted at a single center, therefore the effect of unmeasured and undiscovered confounding variables cannot be ruled out. Second, this study only included Chinese patients, and whether the results can be extrapolated to other ethnic groups needs further study. Third, changes in eosinophil counts, eosinophil derived proteins, or eosinophil chemokines after PCI modulate the inflammatory response after acute cardiac events, which may lead to serious complications, such as stent thrombosis. However, we did not assess these parameters, and in the future work, we should consider analyzing eosinophil-related substances, differential leukocyte counts and their derivatives. Fourthly, due to the retrospective nature of this study and the diagnostic protocol for ACS events, it cannot be guaranteed that all patients had blood samples taken both before and after PCI. Fifthly, this study is unable to discuss the impact of perioperative complications on the outcomes. Before PCI was performed on all patients diagnosed with ACS, patients with surgical contraindications were excluded. The average hospital stay was 3–5 days, and during this period, no cases of coronary artery perforation (CAP) or any other complications, such as bleeding or stroke, occurred from the postoperative stage until discharge, limiting further exploration of perioperative complications in this study. This limitation is worth noting. Finally, eosinophils were only measured at admission, but were not collected at discharge or during follow-up.
Conclusions
High AEC on admission was significantly associated with an increased risk of MACCEs in patients with ACS undergoing PCI, implying the critical role of the AEC in the risk stratification. The introduction of AEC considerably augmented the predictive proficiency of the SYNTAX II score for MACCEs. Larger prospective studies are needed to confirm our findings.
Abbreviations
AEC, absolute eosinophil count; ACS, Acute coronary syndrome; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BMS, bare-metal stent; BRS, bioresorbable scaffold; CAD, coronary artery disease; CV, cardiovascular; CABG, Coronary Artery Bypass Grafting; CHF, congestive heart failure; CVA, cerebrovascular accident; CI, confidence interval; CKD, chronic kidney disease; cNRI, continuous net reclassification improvement; CAP, coronary artery perforation; DBP, diastolic blood pressure; DM, diabetes mellitus; DES, drug-eluting stent; DCB, drug coating balloon; ECP, eosinophil cationic protein; EPX, eosinophil peroxidase; EET, eosinophil extracellular traps; FPG, fasting plasma glucose; GRACE, Global Registry of Acute Coronary Events; HR, Hazard ratio; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein-cholesterol; hs-CRP, high-sensitivity C-reactive protein; IDI, integrated discrimination improvement; IQR, Interquartile range; ICDs, intracardiac defibrillators; IMRS, Intermountain Risk Score; LDL-C, low-density lipoprotein-cholesterol; LVEF, left ventricular ejection fraction; LM, left main coronary artery; LAD, left anterior descending branch; LCX, left circumflex branch; MACCEs, major adverse cardiovascular and cerebrovascular events; MI, myocardial infarction; MBP, major basic protein; NSTEMI, non-ST-segment elevation myocardial infarction; NS, Naples score; NLR, neutrophil to lymphocyte ratio; NOAC, novel oral anticoagulants; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RCA, right coronary artery; ROC, Receiver operating characteristic; SBP, systolic blood pressure; SD, standard deviation; SYNTAX, synergy between percutaneous coronary intervention with taxus and cardiac surgery; STEMI, ST-segment elevation myocardial infarction; SII, systemic immune-inflammation index; TVD, three-vessel lesion; TC, total cholesterol; T2DM, Type 2 diabetes mellitus; TIMI, Thrombolysis in Myocardial Infarction; UA, unstable angina; WBC, white blood cell.
Data Sharing Statement
The unadjusted data that underlies the findings of this article will be accessible by the authors, free from any undue constraints.
Ethics Statement
The studies involving human participants were reviewed and approved by local Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (IRB number: 2016034x). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Statement for Patient Data Confidentiality
We are fully committed to protecting the privacy and confidentiality of our patients’ personal health information. Our organization strictly adheres to all applicable laws, regulations, and ethical standards related to the confidentiality of patient information. We have implemented robust security measures to ensure that patient data is protected from unauthorized access, disclosure, alteration, or destruction. All employees who have access to patient information are required to sign confidentiality agreements and receive regular training on our privacy policies and procedures. We maintain strict access controls to limit access to patient information only to those who need it to perform their job duties. We value the trust that our patients place in us, and we are committed to maintaining the highest standards of confidentiality and privacy protection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFC3602500), National Natural Science Foundation Youth Project (82200405), and Beijing Municipal Health Commission (2022-2-1052, Zhi-Jian Wang).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Aboyans V, Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi:10.1016/S0140-6736(14)61682-2
2. Madjid M, Awan I, Willerson JT, et al. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–1956. doi:10.1016/j.jacc.2004.07.056
3. Toor IS, Jaumdally R, Lip GY, et al. Eosinophil count predicts mortality following percutaneous coronary intervention. Thromb Res. 2012;130(4):607–611. doi:10.1016/j.thromres.2012.05.033
4. Zhao X, Jiang L, Xu L, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol. 2019;26(8):872–882. doi:10.1177/2047487319826398
5. Hayiroğlu M, Çinar T, Çinier G, et al. Evaluating systemic immune-inflammation index in patients with implantable cardioverter defibrillator for heart failure with reduced ejection fraction. Pacing Clin Electrophysiol. 2022;45(2):188–195. doi:10.1111/pace.14436
6. Şaylik F, Çinar T, Selçuk M, et al. Evaluation of Naples Score for Long-Term Mortality in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology;2023. 33197231170982. doi:10.1177/00033197231170982
7. Toor I, Ruckerl D, Mair I, et al. Eosinophil Deficiency Promotes Aberrant Repair and Adverse Remodeling Following Acute Myocardial Infarction. JACC Basic Transl Sci. 2020;5(7):665–681. doi:10.1016/j.jacbts.2020.05.005
8. Xu J, Xiong Y, Tang R, et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovascular Res. 2021;118(9):2165–2178. doi:10.1093/cvr/cvab237
9. Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther. 2013;35(5):563–571. doi:10.1016/j.clinthera.2013.02.022
10. Umemoto S, Suzuki N, Fujii K, et al. Eosinophil counts and plasma fibrinogen in patients with vasospastic angina pectoris. Am J Cardiol. 2000;85(6):715–719. doi:10.1016/S0002-9149(99)00846-2
11. Novotny J, Oberdieck P, Titova A, et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology. 2020;94(22):e2346–e60. doi:10.1212/WNL.0000000000009532
12. Groot H, Van Blokland I, Lipsic E, et al. Leukocyte profiles across the cardiovascular disease continuum: a population-based cohort study. J Mol Cell Cardiol. 2020;138:158–164. doi:10.1016/j.yjmcc.2019.11.156
13. Sasmita B, Zhu Y, Gan H, et al. Leukocyte and its Subtypes as Predictors of Short-Term Outcome in Cardiogenic Shock Complicating Acute Myocardial Infarction: a Cohort Study. Shock. 2022;57(3):351–359. doi:10.1097/SHK.0000000000001876
14. Gudbjartsson D, Bjornsdottir D, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41(3):342–347. doi:10.1038/ng.323
15. S G, Deng Y, Wu J, et al. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis. 2019;286:128–134. doi:10.1016/j.atherosclerosis.2019.05.027
16. Kounis N, Soufras G, Tsigkas G, et al. White blood cell counts, leukocyte ratios, and eosinophils as inflammatory markers in patients with coronary artery disease. Clin Appl Thromb Hemost. 2015;21(2):139–143. doi:10.1177/1076029614531449
17. Zabojszcz M, Januszek R, Siudak Z, et al. Association between the mortality rate and operator volume in patients undergoing emergency or elective percutaneous coronary interventions. Kardiologia polska. 2020;78(2):138–146. doi:10.33963/KP.15123
18. Young J, Peterson C, Venge P, et al. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321(6070):613–616. doi:10.1038/321613a0
19. Acharya K, Ackerman S. Eosinophil Granule Proteins: form and Function. J Biol Chem. 2014;289(25):17406–17415. doi:10.1074/jbc.R113.546218
20. Chihara J, Yamamoto T, Kayaba H. Degranulation of eosinophils mediated by intercellular adhesion molecule-1 and its ligands is involved in adhesion molecule expression on endothelial cells–selective induction of VCAM-1. J Allergy Clin Immunol. 1999;103(5 Pt 2):S452–6. doi:10.1016/S0091-6749(99)70161-2
21. Hogan S, Rosenberg H, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750. doi:10.1111/j.1365-2222.2008.02958.x
22. Verdoia M, Schaffer A, Cassetti E. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J Thromb Thrombolysis. 2015;39(4):459–466. doi:10.1007/s11239-014-1120-3
23. Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109(6):701–705. doi:10.1161/01.CIR.0000116202.41966.D4
24. Joner M, Finn A, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. doi:10.1016/j.jacc.2006.03.042
25. Finn A, Nakazawa G, Joner M, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27(7):1500–1510. doi:10.1161/ATVBAHA.107.144220
26. Ribichini F, Joner M, Ferrero V, et al. Effects of oral prednisone after stenting in a rabbit model of established atherosclerosis. J Am Coll Cardiol. 2007;50(2):176–185. doi:10.1016/j.jacc.2007.03.031
27. Niccoli G, Schiavino D, Belloni F, et al. Pre-intervention eosinophil cationic protein serum levels predict clinical outcomes following implantation of drug-eluting stents. Eur Heart J. 2009;30(11):1340–1347. doi:10.1093/eurheartj/ehp120
28. Park S-J, J-M A, Kim Y-H, et al. Trial of Everolimus-Eluting Stents or Bypass Surgery for Coronary Disease. N Engl J Med. 2015;372(13):1204–1212. doi:10.1056/NEJMoa1415447
29. Serruys P, Morice M-C, Kappetein A, et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N Engl J Med. 2009;360(10):961–972. doi:10.1056/NEJMoa0804626
30. Neumann F, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi:10.1093/eurheartj/ehy394
31. Tam D, Ruel M, Fremes S. Commentary: does the SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery) score even matter? J Thorac Cardiovasc Surg. 2021.
32. Banning A, Serruys P, De Maria A, et al. Five-year outcomes after state-of-The-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. Eur Heart J. 2022;43(13):1307–1316. doi:10.1093/eurheartj/ehab703
33. Hayiroglu M, Keskin M, Uzun AO, et al. Predictive value of SYNTAX score II for clinical outcomes in cardiogenic shock underwent primary percutaneous coronary intervention; a pilot study. Int J Cardiovasc Imaging. 2018;34(3):329–336. doi:10.1007/s10554-017-1241-9
34. Horne B, May H, Muhlestein J, et al. Exceptional mortality prediction by risk scores from common laboratory tests. Am j Med. 2009;122(6):550–558. doi:10.1016/j.amjmed.2008.10.043
35. Çinar T, Şaylik F, Akbulut T, et al. Evaluation of Intermountain Risk Score for Short- and Long-Term Mortality in ST Elevation Myocardial Infarction Patients. Angiology. 2023;74(4):357–364. doi:10.1177/00033197221105753
36. Mert Ilker H, Faysal S, Ahmet Çağdaş Y, et al. Prognostic value of Intermountain Risk Score for short- and long-term mortality in patients with cardiogenic shock. Coronary Artery Disease. 2023;34(2):154–159. doi:10.1097/MCA.0000000000001219
[ad_2]
Source link






