[ad_1]
AAV:PKP2 corrected disease phenotypes in a human iPSC-CM model
To model ARVC disease and identify the molecular, structural, and functional signatures that are fundamental to the disease mechanisms, we carried out RNA sequencing analyses of iPSC-CMs after acute silencing of PKP2 expression. These studies revealed that the desmosome functions as a signaling hub connecting key structures48 in cardiomyocytes such that reduction in PKP2 expression led to down-regulation of structural and functional gene expression encoding components of desmosomes, sarcomeres, intermediate filaments, and ion channels (Fig. 1a). Down-regulation of protein was shown for desmoplakin (DSP), plakoglobin (JUP), myosin-binding protein C3 (MyBPC3), and desmin (DES) (Fig. 1b, the left panel and Supplementary Fig. 1). Trending down-regulation of mRNA was shown for sodium voltage-gated channel α subunit 5 (SCN5A) (Fig. 1b, the right panel). PKP2 deficiency resulted in structural disappearance of PKP2 and DSP from the cellular membrane and caused cell disarray of patterned iPSC-CMs (Fig. 1c). In addition, PKP2 deficiency perturbed both contractile (Fig. 1d) and electrophysiological properties of iPSC-CMs (Fig. 1e).
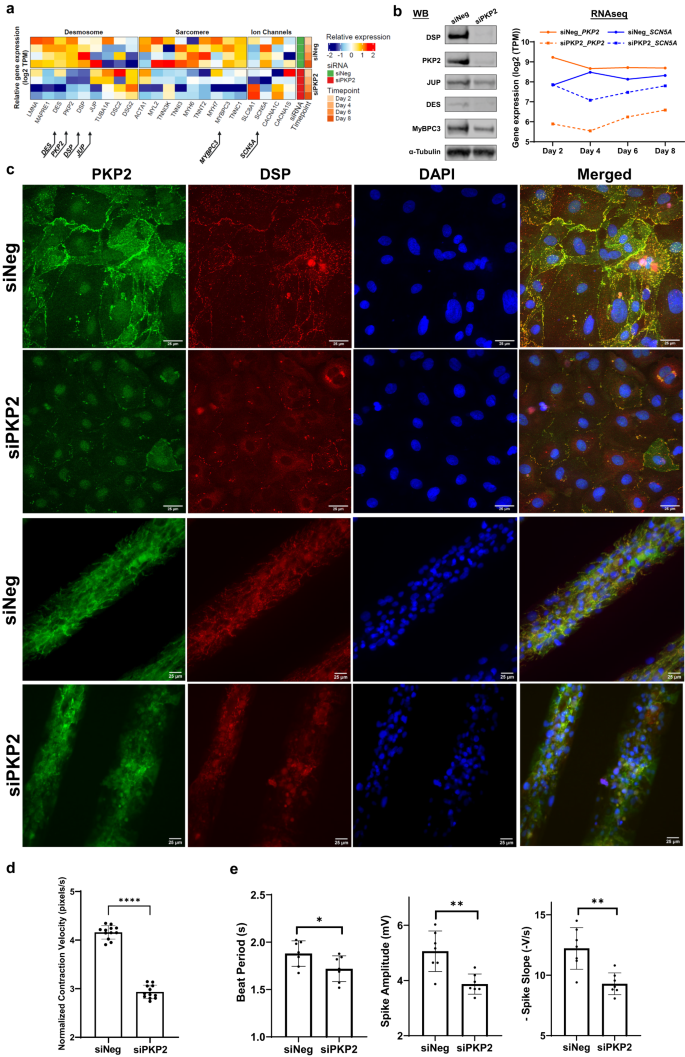
a Heatmap of RNA sequencing analyses from iPSC-CMs (nâ=â1 for negative control, siNeg, and nâ=â3 biological replicates for siRNAs against PKP2, siPKP2) harvested on day 2, 4, 6, and 8, respectively, after siRNA treatment, highlighting effects on genes encoding components of the desmosome, sarcomere, and ion channels. b PKP2 silencing led to reduction in protein expression of DSP, JUP, DES, and MyBPC3 in response to reduced PKP2 protein (Western blot on the left panel, day 8, nâ=â2 biological replicates) and a trending reduction in SCN5A mRNA in response to reduced PKP2 mRNA (RNA sequencing reads from Fig. 1a). c PKP2 silencing resulted in disappearance of PKP2 and DSP protein from the cellular membrane (top two rows, day 10, nâ=â5 technical replicates; IXM confocal microscope; 25âµm) and cell disarray in patterned iPSC-CMs (bottom two rows, day 10, nâ=â3 technical replicates; Leica DMi8 microscope; 25âµm). Immunofluorescent staining: PKP2 in green, DSP in red, and nuclei in blue. d PKP2 silencing led to defective contraction as quantified by contraction velocity using Pulse video analysis (day 3 to 8, nâ=â12 technical replicates for each day, nâ=â2 biological replicates) (Curi Bio)69. Average nuclear counts from live cells were used to normalize contraction velocity. PKP2 silencing led to (e) depressed beat period; depressed amplitude; depressed rate of propagation of electrical signal, detected as extracellular field potential signals from the cardiomyocyte monolayers using Microelectrode array (MEA) plates (day 4 to 10, nâ=â18 technical replicates for each day, nâ=â2 biological replicates) (Axion Biosystems)43. Quantified data were presented as meanâ±âs.d. P value: Studentâs t test. *pâ<â0.05, **pâ<â0.01, ***pâ<â0.001, ****pâ<â0.0001.
The 1st generation expression cassette was used for iPSC-CM-based studies and the 2nd generation for in vivo mouse efficacy studies (Fig. 2a). Dose-dependent protein expression was evident in iPSC-CMs driven by a cardiac-specific troponin T promoter (Fig. 2b and Supplementary Fig. 2). AAV:human PKP2 (AAV:hPKP2), which utilizes an AAV9 variant, CR9-01, and has a higher transduction efficiency to iPSC-CMs than AAV942, restored DSP expression post PKP2 silencing when compared to the reduced DSP protein without AAV rescue (Fig. 2c, d). AAV:hPKP2 restored contractility as quantified by contraction velocity when compared to the reduced contraction velocity without AAV rescue (Fig. 2e). Using human iPSC-CMs as a cell model for ARVC, AAV:hPKP2 restored desmosomes and rescued contractility in PKP2-deficient iPSC-CMs, suggesting PKP2 governs intrinsic cellular properties of cardiomyocytes.
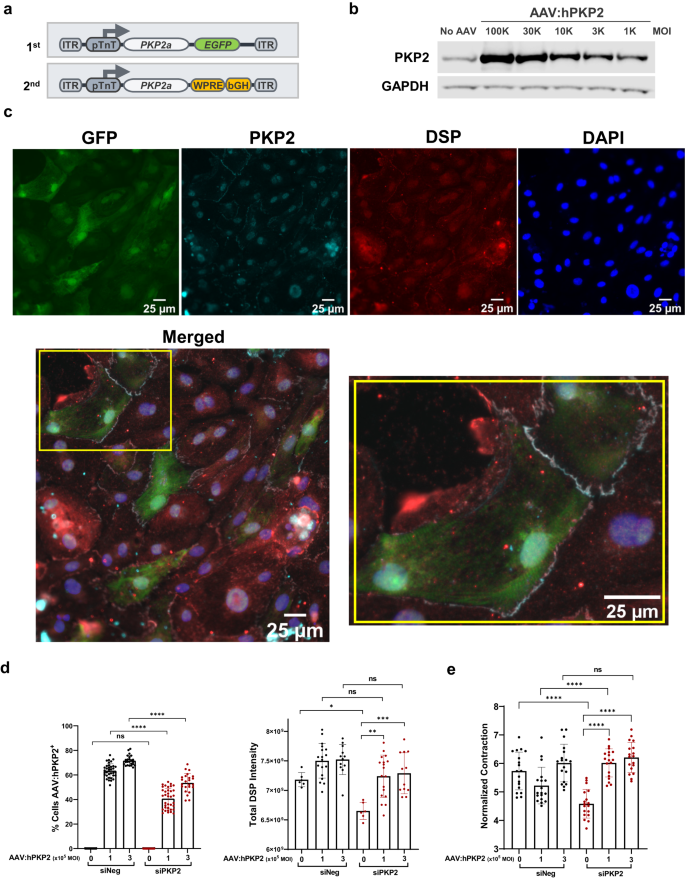
a Schematic representation of the 1st generation and the 2nd generation AAV expression cassette of codon-optimized PKP2a. Key 3â elements in AAV expression cassette include Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and bovine growth hormone polyadenylation signal (bGH). b Western blot analysis showed that the second generation of AAV:hPKP2 is expressed in iPSC-CMs in a dose-dependent fashion by applying viruses at different multiplicity of infection (MOI). c At day 10 of PKP2 silencing and day 8 of AAV transduction, GFP expression of the first generation of PKP2 expression cassette was used to label AAV transduced iPSC-CMs. Codon optimization allows the transgene PKP2 resistant to siRNA-mediated silencing. The immunofluorescent mini panels show cells were stained for GFP, PKP2, DSP and nuclei, respectively, with the bottom large panel showing merged channels (Leica DMi8 microscope; 25âµm). Yellow inset was magnified to highlight two GFP cells expressing transgene PKP2 (gray color) at the junction of each other and at the junction of other non-GFP neighbors. d At day 10 of PKP2 silencing and day 8 of AAV transduction, the left bar graph summarized the percentage of cells without GFP (nâ=â12 technical replicates, nâ=â2 biological replicates) and with GFP (nâ=â24â36 technical replicates, nâ=â2 biological replicates). The right graph showed restored DSP protein expression quantified by total intensity of immunofluorescence signal post PKP2 silencing in the absence (nâ=â6 technical replicates, nâ=â2 biological replicates) or the presence (nâ=â12â18 technical replicates, nâ=â2 biological replicates) of AAV:hPKP2 transgene. Quantified data were presented as meanâ±âs.d. Statistical significance was evaluated by ordinary One-Way ANOVA (Tukeyâs post-hoc test). e AAV:hPKP2 showed rescue of contraction velocity post PKP2 silencing in iPSC-CMs (nâ=â18â27 technical replicates for each day, nâ=â3 biological replicates). Cell contractility was recorded from day 3 to 8 post AAV transduction and analyzed by Pulse video analysis (Curi Bio). Average nuclear counts from live cells were used to normalize contraction velocity. Quantified data were presented as meanâ±âs.d. Statistical significance was evaluated by ordinary Two-Way ANOVA (Tukeyâs post-hoc test). P value: *pâ<â0.05, **pâ<â0.01, ***pâ<â0.001, ****pâ<â0.0001.
Pkp2-cKO ARVC mouse model recapitulated the majority of human
ARVC clinical manifestations
We used a mouse conditional knockout model to assess the feasibility and the efficacy of AAV9-mediated PKP2 gene replacement. Consistent with the early observations of this model31, tamoxifen-induced cardiac deletion of both alleles of Pkp2 in adult mice did not show overt structural and functional changes at 1 week post induction. Tissue collection at the end of the study and weekly monitoring showed disruption of desmosomes and GJs (Fig. 3a and Supplementary Fig. 3), high burden of spontaneous premature ventricular contractions (PVCs) (Fig. 3b) and occurrences of non-sustained ventricular tachycardia (NSVT) (Supplementary Fig. 4), biventricular dilatation (Fig. 3d), and a sharp decline in cardiac function (Fig. 3c) and survival (Fig. 3e) after 3â4 weeks of induced cardiac knock-out of Pkp2. These phenotypes recapitulated human ARVC clinical manifestations. However, unlike in humans, heterozygous disruption of Pkp2 in mouse hearts did not result in cardiac phenotypes that closely recapitulated human ARVC symptoms49,50. Thus, homozygous Pkp2-cKO mouse was used as a model of human ARVC.
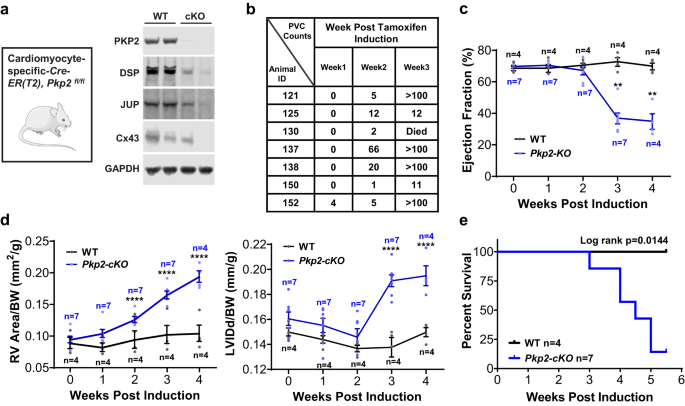
a Pkp2-cKO ARVC mice (αMyHC-Cre-ER(T2), Pkp2fl/fl)31 at ~3 months of age were injected with tamoxifen to induce cardiac knock-out of the Pkp2 gene. Representative immunoblots showed reduction of desmosome proteins PKP2, DSP, JUP, and GJ protein, Cx43. b Pkp2-cKO mice developed spontaneous PVCs as observed during 30âmin of continuous recording of EKG and reported in the table. Statistical evaluation using nonparametric Kruskal-Wallis test with Dunnâs correction showed a significant increase in PVC counts from week 1 to week 3 (pâ=â0.0004). c LV performance measured by % ejection fraction sharply declined at 2 weeks post tamoxifen induction. d Pkp2-cKO mice started to develop biventricular dilatation between 2 and 3 weeks post tamoxifen induction. RV area (left panel) and LV internal diameter end diastole (LVIDd, right panel) were normalized to body weight. e Kaplan-Meier survival curve showed a sharp decline of survival of Pkp2-cKO mice beginning 3 weeks post tamoxifen induction. Animals showed symptoms including sudden death, edema, reduced activity, and reduced tolerance to isoflurane beginning 3 weeks post induction. Quantified data were presented as meanâ±âs.e.m. P value: Ordinary Two-Way ANOVA (Tukeyâs post-hoc test); **pâ=â0.002, ****pâ<â0.0001 vs. WT at 2, 3, and 4 weeks, respectively. Sample size nâ=â4 and 7 for WT and Pkp2-cKO, respectively.
TN-401 or AAV9:mPkp2 treatment largely attenuated disease development and disease progression to mortality in Pkp2-cKO ARVC mouse
To determine whether the AAV9 expression cassette (Fig. 2a, the 2nd generation) encoding either the human PKP2 or the mouse ortholog could counteract the effects of cardiac Pkp2 gene deletion, Pkp2-cKO mice were given a single systemic dose via retro-orbital injection of TN-401 (AAV9: human PKP2 at 3E13 vg/kg) or AAV9:mPkp2 (AAV9: mouse Pkp2 at 5E13 vg/kg) 3 weeks prior to tamoxifen induction of cardiac Pkp2 gene deletion (Fig. 4a). A lower dose level of the human ortholog was selected to limit the risk of overexpressing the human protein in this mouse model. Hankâs Balanced Salt Solution (HBSS) was used as the carrier buffer for TN-401 or AAV9:mPkp2 to prevent aggregation of capsids. It was administered as the vehicle control to WT and to Pkp2-cKO animals. There were 4 experimental groups: âWTâ and âcKOâ were treated with the vehicle and Pkp2-cKO animals treated with either âTN-401â or âAAV9:mPkp2â as shown in Fig. 4.
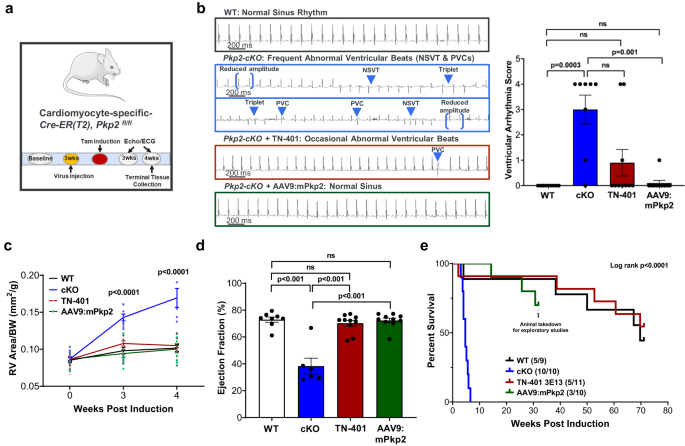
a Study design to evaluate TN-401 or AAV9:mPkp2 efficacy using Pkp2-cKO ARVC mouse model. AAV9 was injected three weeks before gene deletion, TN-401 at 3E13 vector genomes per kilogram bodyweight (vg/kg) and AAV9:mPkp2 at 5E13 vg/kg. Echocardiograph (Echo) and electrocardiogram (EKG) data were collected at week 3 and week 4 post gene deletion. b Raw EKG traces showed a significant contrast in spontaneous arrhythmias in Pkp2-cKO mice in the absence and the presence of TN-401 or AAV9:mPkp2 treatment. PVCs, premature ventricular contractions; NSVT, non-sustained ventricular tachycardia. The right graph summarized arrhythmia scores representing frequency and severity of ventricular arrhythmias. This arrhythmia score is an overall composite score estimating arrhythmia burden (see Supplementary Table 1). Statistical significance in response to TN-401 or AAV9:mPkp2 treatment was evaluated using nonparametric Kruskal-Wallis test with Dunnâs correction. c TN-401 or AAV9:mPkp2 treatment of Pkp2-cKO mice showed efficacy in reducing RV dilation as estimated by RV area normalized to body weight (mm2/g) and (d) maintaining left ventricular ejection fraction at 4 weeks post gene deletion. Time course of RV dilation was evaluated with ordinary Two-Way ANOVA (Tukeyâs post-hoc test), statistical significance shown between the vehicle treated cKO animals and TN-401 treated cKO animals. EF% was evaluated with ordinary One-Way ANOVA (Tukeyâs post-hoc test). e Kaplan-Meier survival curve showed that TN-401 extended median lifespan of Pkp2-cKO mice by â¥58 weeks post gene deletion. Numbers in paratheses showed dead vs live animals by the time of takedown. Animals treated by AAV9:mPkp2 (in green line) were taken down early for exploratory studies. Quantified data were presented as meanâ±âs.e.m. Sample size nâ=â9, 10, 11, 10 for WT, cKO, cKO+TN-401, and cKO+AAV9:mPkp2, respectively.
At 4 weeks post gene deletion and 7 weeks post AAV treatment, human or mouse PKP2 inhibited the development of frequent PVCs and the occurrence of NSVT as summarized by a ventricular arrhythmia score (Fig. 4b, Supplementary Fig. 4, and Supplementary Table 1 used as an overall composite score estimating arrhythmia burden), prevented right ventricular remodeling (Fig. 4c), and prevented decline in left ventricular function (Fig. 4d). Frequent PVCs, RV and LV remodeling, and LV function decline were prominent features of Pkp2-cKO mice at 4 weeks post gene deletion. TN-401 demonstrated significant efficacy in preventing ARVC development and in extending median lifespan by ⥠58 weeks, far beyond the 4.7 weeks observed in the vehicle-treated Pkp2-cKO animals (Fig. 4e). In this same study, we also evaluated efficacy of AAV9:mPkp2 in Pkp2-cKO mice at 3 intervention timepoints and concluded that treatments at 3 weeks before, right after, or 1 week after gene deletion yielded comparable efficacy in EF%, RV remodeling, arrhythmias, and prolonged lifespan of more than 50% of the treated animals by 50 weeks (Supplementary Fig. 5). Overall, these results showed that either the human PKP2 or the mouse ortholog was sufficient to prevent the detrimental cardiac and survival phenotypes of Pkp2-cKO mice when delivered in the AAV9 vector. In addition, we were unable to detect sex difference in either Pkp2-cKO mice or treatment groups (information on sex distribution in each study is detailed in Supplementary Data 1).
To assess the dose response to TN-401 (Fig. 5) or AAV9:mPkp2 (Supplementary Fig. 6), Pkp2-cKO mice were given single systemic treatments via retro-orbital injection of TN-401 at 1E13, 3E13, and 1E14 vg/kg one week after tamoxifen induction of cardiac Pkp2 gene deletion (Fig. 5a). All animals were sacrificed at 4 weeks post induction (3 weeks post AAV treatment) for histological and expression analyses. TN-401 treatment of Pkp2-cKO mice showed dose-dependent efficacy in preventing decline of LV ejection fraction, reducing RV dilation as estimated by RV area normalized to body weight, and a trending reduction in arrhythmias (Fig. 5b). This dose-dependent efficacy was confirmed with larger cohorts of animals in significantly improving LV ejection fraction, reducing RV area and arrhythmia burden, and improving survival (Supplementary Fig. 6).
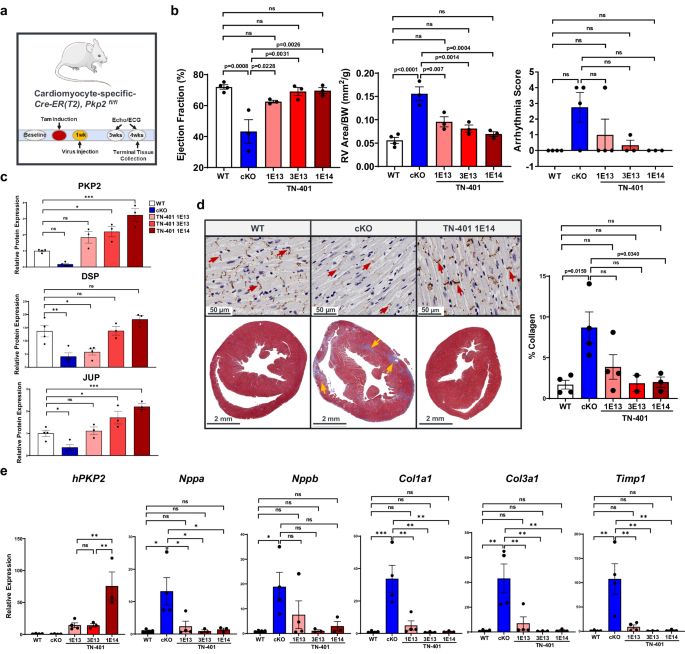
a Study design to evaluate dose-dependent efficacy of TN-401 using Pkp2-cKO mouse model. Mice were injected with TN-401 at 1E13, 3E13, or 1E14 vg/kg at one week after tamoxifen induction of cardiac Pkp2 gene deletion. At 4 weeks post tamoxifen induction (3 weeks post TN-401 injection), animals were sacrificed for expression and histological evaluation. b TN-401 showed dose-dependent efficacy at 3 weeks in preventing decline of % LV ejection fraction, preventing RV dilation (mm2/g), and a trending improvement in arrhythmia scores. Statistical significance of EF% and RV dilation in response to TN-401 treatment was evaluated with ordinary One-Way ANOVA (Tukeyâs post-hoc test) and arrhythmia scores with nonparametric Kruskal-Wallis test with Dunnâs correction. c Semi-quantitative Western blot analyses showed restoration of PKP2, JUP, and DSP protein at 3 weeks post TN-401 treatment. Statistical significance was estimated by ordinary One-Way ANOVA. d Immunohistochemistry for the gap junction protein, connexin 43 (Cx43), in heart tissue sections showed restoration of Cx43 expression at intercalated discs (ID) at 3 weeks post TN-401 treatment (the top panels; 50âµm). Red arrows indicate ID. Trichrome staining showed a significant reduction of fibrosis, muscle (red) and fibrosis (blue), in heart sections at 3 weeks post AAV treatment (the bottom panels; 2âmm). Yellow arrows highlight areas with fibrosis in Pkp2-cKO mouse heart. The percentage of collagen-positive tissue was quantified and shown in the right graph. Statistical significance was estimated by ordinary One-Way ANOVA (Tukeyâs post-hoc test). e RT-qPCR analyses of RV tissue at 3 weeks post TN-401 treatment showed expression of hPKP2 transgene and suppression of heart failure markers (Nppa) (Nppb did not show statistical significance) and fibrosis genes (Col1a1, Col3a1, Timp1). Gapdh was used as internal control. Statistical significance was estimated by ordinary One-Way ANOVA (Tukeyâs post-hoc test). Quantified data were presented as meanâ±âs.e.m. P value: *pâ<â0.05, **pâ<â0.01, ***pâ<â0.001, ****pâ<â0.0001. Sample size nâ=â4, 4, 4, 3, 3 for WT, cKO, cKO+TN-401 at 1E13, 3E13, 1E14 vg/kg, respectively. Two animals died between EKG and echocardiogram recordings, and therefore the cKO and cKO+TN-401 at 1E13 vg/kg has nâ=â3 for echocardiogram parameters.
At molecular level, left ventricle heart tissue showed dose-dependent protein expression of human PKP2 (Fig. 5c, the top panel; Western blot images in Supplementary Fig. 7) as well as corresponding restoration of DSP and JUP, two additional desmosome proteins that were decreased in Pkp2-cKO mice (Fig. 5c, the bottom two panels). Connexin 43 (Cx43), a gap junction protein present at intercalated discs, was reduced in Pkp2-cKO mice, as shown by immunohistochemistry of heart tissue, and was restored in Pkp2-cKO mice treated with TN-401 (Fig. 5d, the top row). TN-401 treatment also significantly reduced fibrosis development and collagen deposition in both right ventricle and left ventricle (Fig. 5d, the bottom row and quantification shown in the right graph). In addition, quantitative analyses of molecular signatures supported that TN-401 treatment reduced mRNA expression of heart failure markers (a significant Nppa reduction and a trending Nppb reduction), fibrosis, and tissue remodeling genes in the right ventricles (only human PKP2 transgene expression was quantified) (Fig. 5e).
Overall, TN-401 or AAV9:mPkp2 treatment supported a dose-dependent efficacy in improving ARVC phenotypes in Pkp2-cKO mouse model of ARVC. TN-401 or AAV9:mPkp2 in the dose-escalation studies demonstrated efficacy at doses â¥3E13 vg/kg in preventing adverse right ventricular remodeling, and improving ventricular function, fibrosis, and electrophysiological properties.
The preventive mode of treatment, dosing before overt structural changes, demonstrated significant benefit of early intervention in largely preventing disease development and extending lifespan. To further examine whether ARVC disease progression could be slowed down or attenuated by restoration of PKP2 expression after overt structural changes, the therapeutic mode of treatment, we dosed animals via retro-orbital injection of AAV9:mPkp2 at 1E14 vg/kg at 2.5 weeks after cardiac deletion of Pkp2 (Fig. 6a). At 2.5 weeks, overt structural changes were observed that coincided with a rapid development of RV dilation, LVEF decline, and significant ventricular arrhythmias (Fig. 3). Note that the rapid mortality presented by this mouse model (within 3â6 weeks of tamoxifen induction) combined with the relatively slow time course of AAV9 transduction and transgene expression make it challenging to perform the therapeutic mode of treatment. However, at 9 weeks post induction, AAV9:mPkp2 prevented further decline of the left ventricle function when compared to the treated animals at 4 weeks (pâ=â0.9416, ns) (Fig. 6c, f), reduced and reversed right ventricle enlargement when compared to the WT level (pâ=â0.6856, ns) (Fig. 6d, g). Arrhythmia scores showed a trending, but not statistically significant reduction (Fig. 6e, h). This therapeutic mode of treatment reduced mortality throughout one year follow-up with a median lifespan by â¥50 weeks (Fig. 6b), which is comparable to the survival benefit observed in the preventive mode of treatment (Supplementary Fig. 6e).
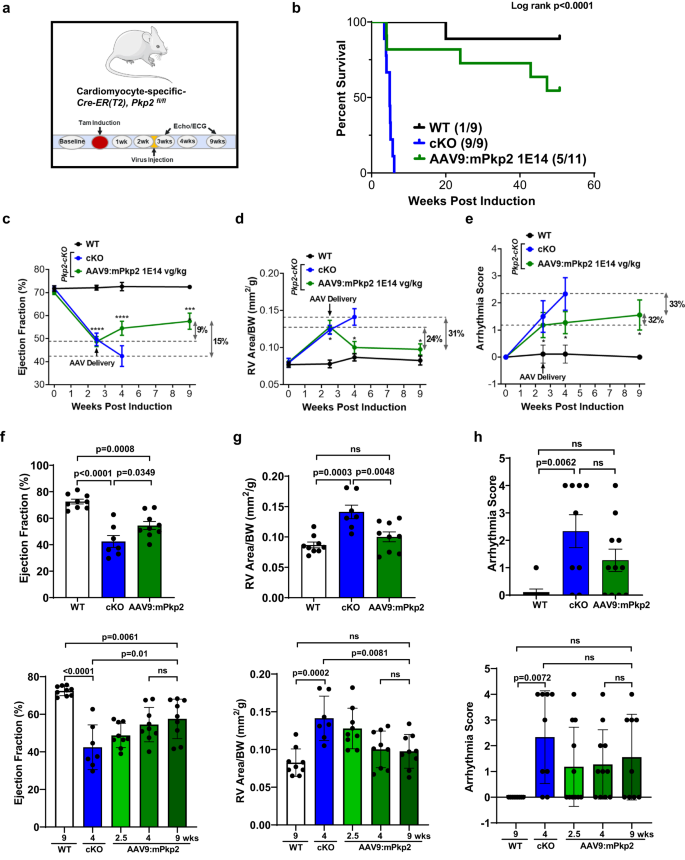
a Study design to evaluate AAV9:mPkp2 efficacy using Pkp2-cKO mouse model with virus injection at 2.5 weeks after Pkp2 cardiac gene deletion by tamoxifen induction. b Vehicle-treated Pkp2-cKO ARVC mice died within 6 weeks of cardiac Pkp2 gene deletion, in contrast, AAV9:mPkp2 treatment at 1E14 vg/kg significantly reduced mortality and extended median lifespan of Pkp2-cKO mice by â¥50 weeks. Numbers in paratheses show dead vs live animals by the time of takedown. AAV9:mPkp2 at 9 weeks post gene deletion and 6.5 weeks post treatment c, f prevented further decline of EF%; d, g reversed right ventricle enlargement and restored RV size similar to that of WT animals; e, h showed a trending reduction in arrhythmias. Statistical significance of EF%, RV area, or arrhythmia scores in time course, c, d, e, was evaluated with ordinary Two-Way ANOVA (Tukeyâs post-hoc test), *pâ<â0.05, ***pâ<â0.001, ****pâ<â0.0001, AAV9:mPkp2 treated vs. WT at 2.5, 4, and 9 weeks, respectively. fâh the top bar graphs show EF%, RV size, and arrhythmia score at 4 weeks post gene deletion and 1.5 weeks post treatment. The bottom bar graphs show multiple comparisons between treatment groups at different time points. P value for all bar graphs in fâh: statistical significance of EF% or RV area was evaluated with ordinary One-Way ANOVA (Tukeyâs post-hoc test) and arrhythmia scores with nonparametric Kruskal-Wallis test with Dunnâs correction. All quantified data were presented as meanâ±âs.e.m. Sample size nâ=â9, 9, 11 for WT, cKO, and cKO+ AAV9:mPkp2 at 1E14 vg/kg, respectively. Four animals died between EKG and echocardiogram recordings at 4 weeks, and therefore cKO and cKO+AAV9:mPkp2 had nâ=â7 and nâ=â9 for echocardiogram, respectively.
Restoration of PKP2 expression led to a highly coordinated and durable correction of PKP2-associated transcriptional networks beyond desmosomes
It was rather surprising to observe that restoration of a single desmosome component, PKP2, led to significant survival benefits, improved cardiac function, reversed adverse RV remodeling, reduced ventricular arrhythmia frequency and severity, and prevented fibrosis. We asked whether âon-targetâ PKP2 effects possibly extend beyond its effects on the desmosome by evaluating PKP2 dose-dependent response, specifically at the transcriptional level. To our knowledge, there has been no reported study that reveals whether (1) PKP2 dynamically coordinates its gene expression with other desmosome members, and (2) to what extent PKP2 quantitively dictates the state of disease progression. To obtain a deeper understanding, two large-scale RNA sequencing analyses were conducted.
Pkp2-cKO mice were given a single systemic dose via retro-orbital injection of TN-401 at 3E13 or 6E13 vg/kg one week before tamoxifen induction of cardiac Pkp2 gene deletion (Fig. 7a) and cardiac function and arrhythmias were evaluated at 4 and 9 weeks post induction. Mice were sacrificed at 9 weeks post induction and heart tissues were collected for RNA sequencing and quantification of PKP2 RNA and protein expression. At a 2-fold expression difference between 3E13 vg/kg and 6E13 vg/kg doses at 9 weeks (Fig. 7b and Supplementary Fig. 8), we did not observe significant dose-dependent difference in key readouts of EF%, LV mass, RV dilatation, and arrhythmia score, although one out of six animals at the high dose vs 5 out of nine animals at the low dose had arrhythmia scores â¥1 at 9 weeks post induction (Fig. 7c). We decided to evaluate specific gene classes including desmosome, gap junctions (GJs), sarcomere, ion channels and Ca2+ handling systems, heart failure markers, and fibrosis, that have been previously demonstrated to be significant contributors to disease mechanisms (Fig. 7d)24,29,30,31,32,33,34,35,36,37. Comparison between WT vs vehicle treated Pkp2-cKO animals showed significant changes in gene expression in these classes and an extensive reversal of these changes in response to TN-401 (Fig. 7e, genes of interest marked in red). Intriguingly, RNA sequencing analysis at the transcriptional level showed a positive dose correlation to TN-401 among structural genes encoding desmosomes, Cx43, sarcomeres, ion channels and Ca2+ handling proteins (Fig. 7f). When examining expression of heart failure markers and fibrosis genes, we noticed a negative dose correlation to TN-401 (Fig. 7f). Therefore, while key functional readouts of efficacy could not be distinguished between dose levels of 3E13 and 6E13 vg/kg, the 2-fold difference in PKP2 transcript levels achieved by these two doses did result in quantitative and dose-dependent changes in transcriptional signatures described above. Based on this observation, we believe that identification of key genes can be informative in associating a transcriptional signature with a particular phase of ARVC disease progression and therefore, may facilitate patient stratification in a more quantitative and precise manner, particularly in early âconcealedâ phase when structural changes are not evident.
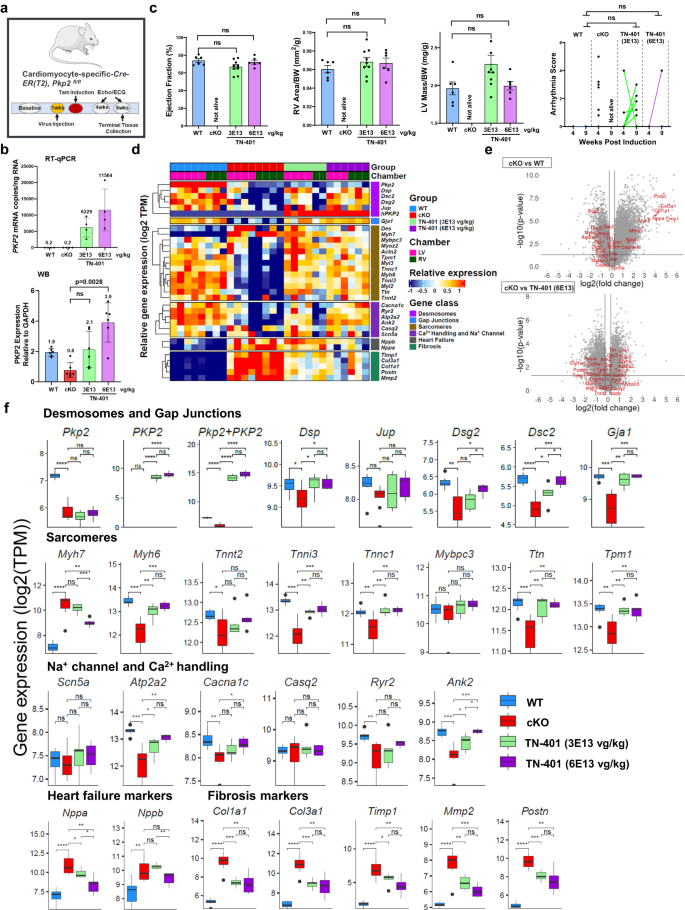
a Study design to evaluate TN-401 dose-dependent efficacy at week 4 and 9 post tamoxifen induction of Pkp2 gene deletion in Pkp2-cKO mouse model. Animals were dosed by TN-401 at 3E13 or 6E13 vg/kg at 1 week before induction. b Absolute human PKP2 transgene mRNAs at two doses were quantified in copy numbers per ng of total LV RNA using a mRNA standard (RT-qPCR, the top panel). Endogenous mouse Pkp2 mRNA is not detected by human-specific primers and probes. No statistical comparison was performed between mouse and human gene expression. Human PKP2 transgene protein levels at two doses were compared to the endogenous level of mouse PKP2 protein in WT or Pkp2-cKO mouse post cardiac gene deletion by Western blot analysis (WB, the bottom panel). c TN-401 treatment at 3E13 or 6E13 vg/kg at week 9 post gene deletion showed comparable efficacy in EF%, RV area (mm2/g, normalized to body weight), LV mass (mg/g, normalized to body weight), and arrhythmia scores. Statistical significance of EF% or RV area was evaluated with ordinary One-Way ANOVA (Tukeyâs post-hoc test) and 4 to 9-week arrhythmia scores with ordinary Two-Way ANOVA (Tukeyâs post-hoc test). Quantified data were presented as meanâ±âs.e.m. d Heatmap of gene expression was sorted by treatment groups, heart chambers (LV vs RV), and gene classes. Values were presented in scaled log2-transformed. e Volcano plots from differential gene expression (DGE) analysis showed changes between cKO vs WT (WT as reference) (the top graph) and TN-401 at 6E13 vg/kg vs cKO (cKO as reference) (the bottom graph). The X-axis represented log 2 of fold change in gene expression. The Y-axis showed the negative log 10 of p values obtained from DGE analysis for each gene. Genes highlighted in red were selected from each gene class shown in (d). f Boxplots showed group-wise gene expression for each representative gene of the selected gene classes. Each box showed the distribution of expression values in the following manner: the midline represented the median expression value, the box indicated the interquartile range where the middle 50% of values lie, and the whiskers at the top and bottom of each box represented the range of values outside the interquartile range. The black dots represent values that fall outside the 2nd and 3rd quartiles. Values were log 2 of TPM (Transcripts Per Million) and were aggregated from LV and RV. Comparison p values were calculated by Studentâs t test: p values: *pâ<â0.05, **pâ<â0.01, ***pâ<â0.001, ****pâ<â0.0001. Sample size nâ=â6, 8, 9, 6 for WT, cKO, cKO+TN-401 at 3E13 and 6E13 vg/kg, respectively.
Transcriptome analyses showed that TN-401 restored expression of structural genes and attenuated expression of genes encoding adverse remodeling factors in a highly coordinated and quantitative fashion. We asked whether such transcriptional response can be sustained to attenuate disease progression and therefore, extend survival over a longer duration.
As shown earlier in Fig. 6, a single dose of AAV9:mPkp2 treatment at 1E14 vg/kg after overt cardiomyopathy halted disease progression via reversed adverse right ventricular remodeling, improved LV function, a trending reduction in arrhythmias, and extended median lifespan by ⥠50 weeks post induction of Pkp2 deletion. Heart tissues collected at 51 weeks post induction of Pkp2 deletion were analyzed by RNA sequencing (Fig. 8a). Compared to intervention before overt structural changes (the preventive mode, animals dosed at 1E13, 3E13 or 1E14 vg/kg), AAV9:mPkp2 intervention after overt structural change (the therapeutic mode, animals dosed at 1E14 vg/kg) showed comparable efficacy in extending life span at the same dose, 1E14 vg/kg (Fig. 8b). PCA51 showed that transcriptional profiles of AAV9:mPkp2-treated Pkp2-cKO animals were clustered close to WT and distant from vehicle-treated animals, suggesting a normalization of transcriptional landscape close to WT in response to the treatment (Fig. 8c). While the transcriptional profile of low-dose treated animals showed a partial recovery pattern, the transcriptional profiles of the high-dose treated animals effectively overlapped with that of WT samples (Fig. 8c). In addition, when comparing the total number of differentially up- or down-regulated genes relative to the WT animals, the preventive mode and to a lesser degree, the therapeutic mode of intervention showed a significant normalization compared to that between Pkp2-cKO and WT animals (Fig. 8d). When comparing vehicle-treated Pkp2-cKO animals vs WT, the significant negatively enriched gene sets identified by Gene Set Enrichment Analysis (GSEA)44 were mitochondrial dysfunction, cardiac muscle contraction, and cardiac muscle conduction. The top significant positively enriched gene sets were predominantly fibrosis related. Both modes of intervention showed significant reversal of these enriched gene sets with the preventive mode supporting the most complete reversal (Fig. 8e). To our surprise, the long-term survival benefit offered by either mode of intervention was supported by a broad spectrum of sustained correction of gene expression encoding components of the desmosomes, sarcomeres, ion channels and calcium handling systems, along with multiple pathways that regulate metabolism, fibrosis, inflammation, and apoptosis as shown (Fig. 8f, g). Once again, both modes of intervention showed significant reversal of these enriched gene sets with the preventive mode effect being most complete (Fig. 8g). Quantitative RT-PCR validated that at the same dose, 1E14 vg/kg, each mode of intervention maintained a similar level of Pkp2 transgene expression at 51 weeks, suggesting the mode of intervention does not change the durability of the transgene expression (Fig. 8h). Expression of fibrosis genes (Timp1, Col1a1, and Col3a1) were significantly lowered by both modes of treatments at 1E14 vg/kg except for Col3a1 in left ventricle in response to the therapeutic mode of treatment. Expression of heart failure genes (Nppa and Nppb) showed trending responses to the preventive mode of treatment relative to the untreated cKO animals (Fig. 8h). In agreement with the observation shown by RNA-seq analyses, fibrosis or heart failure genes were reduced to a lesser extent in therapeutic mode than in the preventive mode among age-matched animals (Fig. 8f, g the top panel).
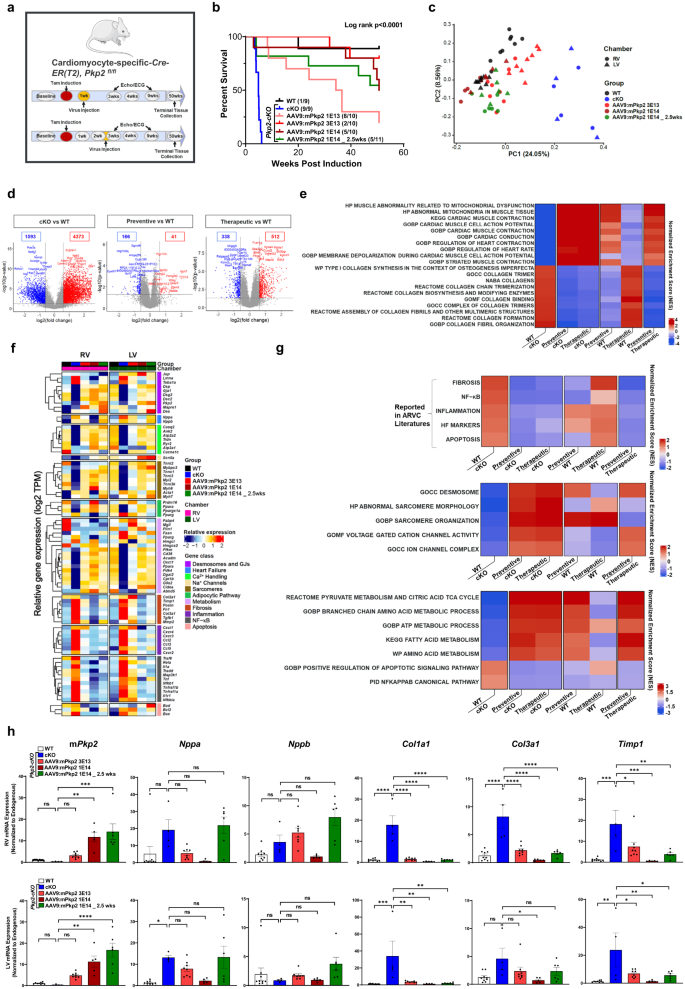
a Study design to evaluate AAV9:mPkp2 efficacy in reducing mortality at 51 weeks post tamoxifen induction of Pkp2 deletion in Pkp2-cKO mouse model. AAV9:mPkp2 was dosed at 1E13, 3E13, and 1E14 vg/kg either 1 week before the induction (the preventive mode of treatment) or at 1E14 vg/kg at 2.5 weeks after induction (the therapeutic mode of treatment). b Kaplan-Meier curve showed percent survival for each mode of treatment for 51 weeks post Pkp2 deletion. Numbers in paratheses show dead vs live animals by the time of takedown. c Principal Component Analysis (PCA) showed clusters of gene transcripts from WT (animals taken down at 51 weeks post induction), vehicle treated Pkp2-cKO animals (animals taken down at 4 weeks post induction), and AAV9:mPkp2 treated animals (animals taken down at 51 weeks post induction). Principal components 1 and 2 were visualized in X and Y axes. Numbers in paratheses represented variation in the data explained by each PC. d Volcano plots from differential gene expression analysis showed changes between cKO vs WT, preventive vs WT, and therapeutic vs WT. Numbers in boxes represented down-regulated and up-regulated genes in blue and red, respectively. The X-axis represented log 2 of fold change in gene expression. The Y-axis showed the negative log 10 of p values obtained from DGE analysis for each gene. e Top 10 positively and top 10 negatively enriched cardiac gene sets were shown with FDR Q value less than 0.25 in Gene Set Enrichment Analysis (GSEA) of Pkp2-cKO vs WT (the far-left column). These enriched gene sets were used to compare preventive vs Pkp2-cKO, therapeutic vs Pkp2-cKO, preventive vs WT, therapeutic vs WT, and preventive vs therapeutic. f Relative gene expression of selected genes was measured by RNA-seq. Samples were sorted by treatment groups and RV and LV chambers. Genes were categorized by gene classes. Each column depicted a scaled average across samples of each treatment group. Number of animals in each treatment group used for RNA sequencing were 9 WT, 4 cKO, 2 at 1E13 vg/kg (not included on the Heatmap), 8 at 3E13 vg/kg, 5 at 1E14 vg/kg, and 6 at 1E14 vg/kg (the therapeutic mode) with both RV and LV collected. g GSEA heatmap presented positively and negatively enriched cardiac gene sets in Pkp2-cKO vs WT. These enriched gene sets were used to compare preventive vs Pkp2-cKO, therapeutic vs Pkp2-cKO, preventive vs WT, therapeutic vs WT, and preventive vs therapeutic. The top heatmap showed known gene sets reported in ARVC literatures (Fig. 7d); middle and bottom heatmaps showed annotated gene sets of Canonical Pathways and Gene Ontology groups from Human MSigDB database (v2023.1.Hs) and had Q valueâ<â0.25 (See Methods). h RT-qPCR analyses showed expression of a total of mouse Pkp2 mRNA (including mouse transgene mRNA), heart failure marker genes, Nppa, Nppb, and fibrosis genes, Timp1, Col1a1, and Col3a1, in RV (top row) and LV (bottom row) at 51 weeks post Pkp2 deletion. Statistical evaluation was performed using ordinary One-Way ANOVA (Tukeyâs post-hoc test); P values: *pâ<â0.05, **pâ<â0.01, ***pâ<â0.001, ****pâ<â0.0001. Sample size nâ=â9, 9, 10, 10, 10, 11 for WT, cKO, cKO+AAV9:mPkp2 at 1E13, 3E13, and 1E14 vg/kg 1 week post cKO induction, cKO+AAV9:mPkp2 at 1E14 vg/kg 2.5 weeks post cKO induction, respectively.
We concluded that long-term restoration of PKP2 expression by gene replacement approach was correlated with sustained restoration of a broad spectrum of structural genes and pathways, supporting a notion that early intervention is the key to restoring PKP2-associated intrinsic transcriptional networks and their functions and therefore, increasing overall cardiomyocyte fitness to effectively mitigate adverse maladaptive remodeling such as fibrosis as early as possible. These results strongly support that PKP2-associated transcriptional networks can be used to quantitatively evaluate the extent of disease progression and gene therapy efficacy at the molecular level.
More than 10Ã an efficacious dose of TN-401 proved to be tolerated in WT CD1 mice
A six-week pilot tolerability study of TN-401 via intravenous injection at 1E14 or 3E14 vg/kg in WT CD1 mice (Fig. 9a) showed no adverse effects at â¥10x an efficacious dose on body weight (Fig. 9b), heart weight and ventricular functions (Fig. 9c), neutrophil to lymphocyte ratio (Fig. 9d), liver weight and enzyme levels (Fig. 9e), and platelet count and hemoglobin levels (Fig. 9f). Histological analyses showed no TN-401-related changes in heart, lung, liver, pancreas, brain, kidneys, and skeletal muscle examined. Pivotal IND enabling toxicology studies conducted by Tenaya Therapeutics demonstrated safety of TN-401 in both mice and non-human primates. Due to the focus of this report, these data are not included, but were contained in the TN-401 IND application, which has received clearance from the FDA.
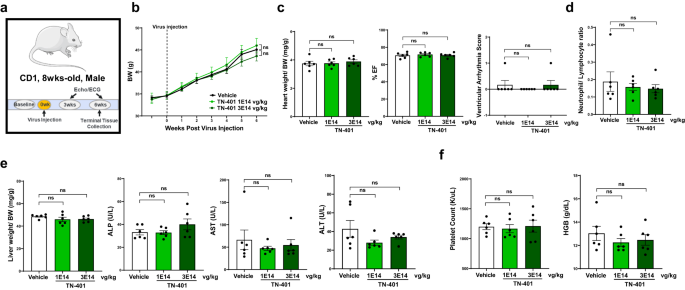
a Study design to evaluate TN-401 tolerability in WT CD1 mice. Mice were injected with TN-401 at 1E14 and 3E14 vg/kg, respectively, after baseline readings of body weight, echocardiography, and EKG. Readings post virus injection were recorded at 3 and 6 weeks, respectively, including echocardiography and 30-min ECG. Mice were sacrificed in week 6 and tissues and blood samples were collected. b Body weight progression for 6 weeks. c Heart weight normalized to body weight, %EF, and ventricular arrhythmia score at 6 weeks. d Neutrophil to lymphocyte ratio at 6 weeks. e Liver weight normalized to body weight and live function tests, alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT) at 6 weeks. f Platelet counts and hemoglobin (HGB) amount at 6 weeks. P value: statistical significance of BW progression at 6 weeks was evaluated with ordinary Two-Way ANOVA; heart weight/ bodyweight, EF%, neutrophil to lymphocytes ratio, liver weight/body weight, ALP, AST, ALT, platelet counts, or HGB were evaluated with ordinary One-Way ANOVA (Tukeyâs post-hoc test); and arrhythmia scores with nonparametric Kruskal-Wallis test with Dunnâs correction. All quantified data were presented as meanâ±âs.e.m. Sample size nâ=â6 for all groups.
[ad_2]
Source link