[ad_1]
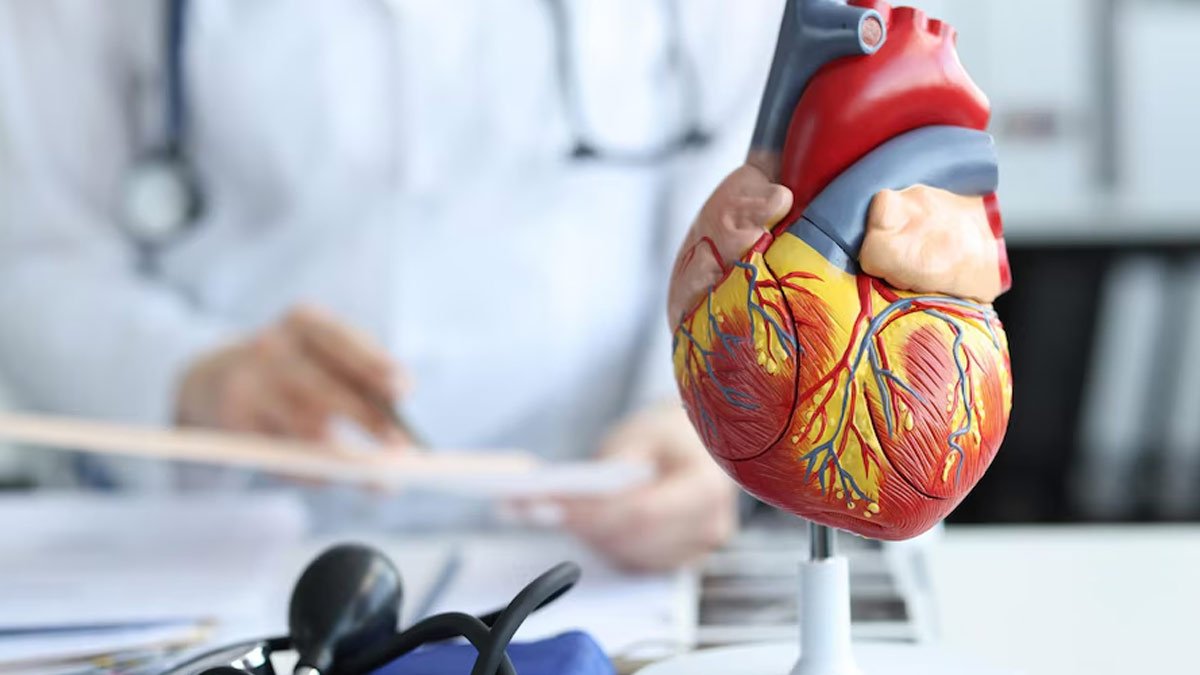
The U.S. Food and Drug Administration (FDA) has approved the weight loss drug Wegovy (semaglutide) to reduce the risk of cardiovascular death, heart disease, and stroke in adults who are overweight or obese. The agency says the shot should be used “in addition to a reduced-calorie diet and increased physical activity.”
“This patient population is at increased risk of cardiovascular death, heart attack, and stroke. Providing treatment options proven to reduce this cardiovascular risk is a major advance for public health,” he said. added.
Wigoby, which contains the same active ingredient as the type 2 diabetes drug Ozempic, was already approved by the FDA in 2021 for weight loss in overweight or obese adults with at least one other weight-related disease, such as type 2 diabetes and hyperemia. approved by. High blood pressure or high cholesterol.
In a high-profile late-stage clinical trial published in November 2023, Wegovy reduced the risk of heart disease-related death, heart attack, and stroke by 20 percent compared to a placebo in overweight or obese adults with heart disease. Ta.
Demand for these new weight loss drugs has exceeded supply for several months. However, Novohe Nordisk, which manufactures both Wigoby and Ozempic, said it would begin gradually increasing its supply of Wigoby starter doses this year. This new approval could make it easier for more people to get insurance, which is sure to further increase demand.
This is breaking news and will be updated.
[ad_2]
Source link