[ad_1]
Introduction
Older adults usually have poor living ability and are prone to falls and fractures, with hip fractures being one of them. Hip fractures are often considered the ultimate fracture in older adults due to their high mortality and disability rates,1 what else, it may lead to many complications.
Heart failure is a common complication after hip fracture. Research has shown that heart failure is an independent risk factor for mortality at least 1 year of follow-up in older patients with hip fractures.2 Two studies in 2020 and 2021 have shown that heart failure has a negative impact on the improvement of daily activity ability in older patients with hip fractures3,4 In 2021, You et al investigated the risk factors for postoperative heart failure in older patients with hip fractures, but lacked the establishment of relevant prediction model.5 In 2023, Tian et al studied the risk factors for perioperative heart failure in older patients with hip fractures and established a prediction model, but did not distinguish between preoperative and postoperative heart failure, and lacked calibration and decision curves.6 What else, there many studies about perioperative risk factors for patients combined with coronary artery disease undergoing noncardiac surgery,7–9 but there is less about hip fractures’ surgery, so it is necessary for us to construct a more integrated prediction model for preventing the AHF in hip fracture patients with CHD.
95% hip fractures are treated surgically. Hip fracture surgery can achieve painless stability of the lower limbs and restore patients to their pre-fracture functional state. However, for older patients with multiple comorbidities, especially those with CHD, the surgical process and anesthesia risks often have a secondary impact on the older patients, leading to increased risk. Therefore, studying the characteristics of postoperative heart failure can take into account the impact of the surgical procedure, which is helpful for clinical practice.
So far, there is no appropriate risk score for postoperative AHF in older hip fracture patients combined with CHD. And it is our aim to construct a prediction model about it. Through this model, it can provide a personalized decision-making tool for clinicians more effectively, aiming to identify and reduce the occurrence of this disease as soon as possible.
Materials and Methods
Patients and Groups
This study retrospectively collected data on 1288 older individuals between 65 and 95 years of age who were diagnosed with CHD and had sustained hip fractures from January 2017 to December 2021. Among them, 214 patients with multiple fractures, pathological fractures, old fractures, conservative treatment, chronic heart failure, chronic atrial fibrillation, and incomplete clinical data were excluded. We divided the remaining 1074 patients them into a training set (n=808 from January 2017 to December 2020) and a validation (n=266 from December 2020 to December 2021). The database is the Elderly Orthopedic electronic medical record system of the Third Hospital of Hebei Medical University. The indicators we collected include basic demographic characteristics of patients: age, gender, fracture type, injury mechanism, comorbidities; Laboratory indicators or imaging examination results, such as hemoglobin, albumin, electrolyte, carotid artery plaques, coronary calcification and left ventricular ejection fraction; Surgical-related indicators: preoperative waiting time, intraoperative bleeding volume, anesthesia method, history of CHD: taking antiplatelet or anticoagulant drugs, revascularization condition. Our research was ethically approved by the Ethics Review Committee of the Third Hospital of Hebei Medical University, and informed consent was waived due to the use of a retrospective design.
Disease Definition
The definition of AHF is determined based on the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. AHF is acute or aggravated left heart dysfunction leads to sudden decrease in cardiac output, increased pulmonary circulation pressure, and increased peripheral circulation resistance, with left heart failure being the most common symptom, which often need the patient to seek urgent medical attention such as Oxygen inhalation, ECG monitoring, intravenous injection cardiotonic, diuretic, and vasodilator. Clinical laboratory examinations that need to be considered include lung X-ray (to determine the presence of pulmonary edema), electrocardiogram (to rule out arrhythmias and acute coronary syndrome), and echocardiography (to determine the presence of cardiac structure, systolic and diastolic issues). Comprehensive evaluation of BNP values and patient clinical manifestations (wheezing, lower limb edema, auscultation of wet rales). Acute heart failure often has high mortality, in-hospital mortality ranges from 4% to 10%.10
Statistical Analysis
We use SPSS 26.0 and R language software as our statistical analysis software. We use means and standard deviations (SD) to represent continuous variables, while use absolute numbers and percentages to represent categorical variables. The Student’s t-test or the Mann Whitney u-test were used to compare the continuous variables. The chi-square test or Fisher’s exact test was used to compare categorical variables. In the training set, based on whether AHF occurred, patients were divided into heart failure group and non-heart failure group, and the two groups were compared to find significant differences. We included indicators with significant differences (p<0.05) in univariate and multivariate binary logistic regression analysis by enter method to identify independent risk factors for AHF. The significance of the correlation was used by odds ratios (OR) and 95% confidence intervals (CI). The Hosmer Lemeshow test’s result p>0.05 show that this nomogram prediction model has great fitness. The nomogram prediction model for postoperative AHF was established based on the multivariate logistic regression analysis results. The discriminative ability of the prediction model was based on AUC of the receiver operating characteristic curve. We use the calibration curve to evaluate the predicted and actual probabilities of this prediction model. We use decision curve analysis to evaluate the clinical application value of the prediction model.
Results
Characteristics of Older Hip Fracture Patients with or Without Acute Heart Failure
Figure 1 was the flow chart of our research. We included 1288 hip fracture patients combined with CHD age range from 65 to 95 in our research, and after excluding 214 patients who did not meet the incorporation criteria, the remaining 1074 patients were enrolled in our study. We divided them into the training set (patients included from 2017 to 2020, n=808) and the validation set (patients included from 2020 to 2021, n=266). In the training set, 346 patients experienced postoperative heart failure, and these patients were included in a univariate and multivariate analysis. Table 1 is the demographic and clinical characteristics of older patients with or without AHF in the training set. Most of the patients were women, and they accounted for 58.5%. Four hundred and thirty-seven (54.1%) patients had intertrochanteric fracture and 371 patients had femoral neck fracture, which accounted for 45.9%. A total of 252 (31.2%) patients had antiplatelet or anticoagulants drugs before and 69 (8.5%) patients previously receive revascularization. The average left ventricular ejection fraction of these patients was 61.83%. Accidently fall is the most common cause of hip fractures, accounting for 78.2%, car accident and fall by dizziness accounted for only 11.2% and 10.5%. Hypertension was the most common comorbidity, accounting for 61.3%, while diabetes and cerebrovascular disease accounted for 40.8% and 43.1%. Fifty-nine patients have an ASA rating ≥3 at admission. Five hundred and twenty patients with preoperative waiting time >3 days. The average hemoglobin at admission was 104.47g/L, and the average albumin at admission was 32.13g/L. The average intraoperative bleeding volume was 211.31mh. Three hundred and seventy-five (46.1%) patients had anemia at admission, and 240 (29.7%) patients had hypoproteinemia at admission.
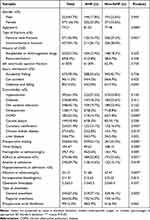 |
Table 1 The Demographic and Clinical Characteristics of Older Patients with or without Acute Heart Failure (AHF) |
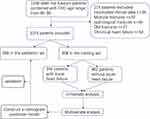 |
Figure 1 The flowchart of our research. |
Univariate and Multivariate Analysis of Risk Factors for Acute Heart Failure and Construct a Nomogram Prediction Model
Univariate and multivariate logistic analysis of risk factors for AHF in the training set is shown in Table 2. From the table, we can find that in univariate analysis, there were significant statistical differences in fracture type, age, coronary calcification, ASA grade ≥3, concomitant COPD, anemia at admission, chronic kidney disease, hypoproteinemia at admission, and preoperative waiting time >3 days. Incorporating these factors into multivariate analysis reveals significant factors such as fracture type, age, ASA grade ≥3, concomitant COPD, anemia on admission, and preoperative waiting time >3 days. We incorporate these risk factors to construct a nomogram prediction model as shown in Figure 2.
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis of Risk Factors for Acute Heart Failure in Training Set |
 |
Figure 2 The nomogram prediction model for postoperative acute heart failure. |
ROC Analysis and Verification by a Calibration Curve and Decision Curve Analysis
Figure 3 is the ROC curve of nomogram and other risk factors, we can see that the nomogram has better discrimination ability than other factors, Figure 4A is the ROC curve of nomogram prediction model in the training set, the AUC of the nomogram prediction model is 0.778 (95%Cl 0.746–0.811). Figure 4B is the ROC curve of the validation set, the AUC of it is 0.748 (95%Cl 0.687–0.810). The calibration curve of the training set and the validation set is shown in Figure 5 and with Hosmer Lemeshow goodness of fit test shows that it is well calibrated (p > 0.05). Decision curve analysis in Figure 6 shows that the nomogram prediction model has good clinical benefits.
 |
Figure 3 The receiver operating characteristic curves of nomogram and different risk factors. |
 |
Figure 4 (A) The receiver operating characteristic curves of nomogram in training set. (B) The receiver operating characteristic curves of patients in validation set. |
 |
Figure 5 (A) The calibration curve of the nomogram in the training set. (B) The calibration curve of the in the validation set. |
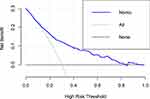 |
Figure 6 The decision curve analysis of the nomogram in the training set. |
Discussion
Our study found that patients with hip fractures combined with CHD have a higher incidence of postoperative heart failure, reaching 42.8%. Fracture type, anemia at admission, ASA ≥ 3, surgical delay time > 72 hours, and combined with COPD are risk factors for postoperative heart failure. And the anesthesia type, previous CHD treatment and preoperative atrial fibrillation do not affect postoperative AHF. We construct a nomogram prediction model and the nomogram prediction model has great discrimination ability.
A review in 2014 mentioned that the incidence rate of ischemic heart disease or heart failure during the perioperative after hip fracture is 35% −42%.11 In our research, the incidence rate of postoperative AHF was 42.8%. Our result is higher than other related studies, which may be related to the combination of CHD in the patients we included. There was no significant statistical difference in the outcome between all CHD patients who had previously taken antithrombotic drugs or had a history of revascularization. A previous study on intraoperative hypotension and postoperative outcomes in patients with coronary artery disease showed that the severity of coronary vessels does not affect the occurrence of cardiovascular events after noncardiac surgery,12 which is partially similar to our results. And we think that patients who underwent revascularization had relatively severe ischemic symptoms before, but they were able to recover well after revascularization and patients who had not undergone revascularization before may experience continued progression of coronary artery stenosis, leading to increased risk. Patients who receive platelet or anticoagulant therapy will stop using it for a period of time before surgery, so whether to take antiplatelet or anticoagulant therapy before will not have a significant difference on postoperative heart failure. In our research results, the average hemoglobin level in the postoperative heart failure group was 99.62g/L, the incidence of anemia is 46.1%. Previous studies have shown that preoperative anemia in patients with hip fractures often indicates weakness and poor postoperative functional recovery.13 Additionally, preoperative anemia is associated with postoperative heart failure, pneumonia, and cerebrovascular disease,14 which is similar to our research findings. Anemia can reduce organ tolerance to ischemia by limit the supply of oxygen to terminal organs and tissues. The decrease in hemoglobin caused by anemia can also cause cell hypoxia, resulting in dysregulation of intracellular homeostasis, decreased concentration of adenosine triphosphate, and finally cell death.15,16 Our results also found that compared to femoral neck fractures, intertrochanteric fractures was a risk factor. The impact of fractures on postoperative AHF can also be explained by anemia. Patients with intertrochanteric fractures often experience more bleeding compared to those with femoral neck fractures. A research in 2019 mentioned that due to blood leakage into the tissue chamber and stress hemolysis, intertrochanteric fractures often have occult blood loss. The occult blood loss occurs in more than 80% of preoperative intertrochanteric fractures, and the blood that seeps into the tissue, resulting in a decrease of blood volume and hemoglobin.17 For patients with anemia or intertrochanteric fracture, measures such as blood transfusion and nutritional supplementation should be taken before surgery, and blood routine indicators should be reviewed timely.
Heart failure and COPD often coexist. It is our conclusion that combine with COPD can contribute to postoperative AHF. Patients with COPD often experience chronic hypoxia, which can lead to pulmonary artery vasoconstriction and vascular remodeling, this ultimately leads to increased pulmonary vascular resistance and impaired cardiac systolic and diastolic function.18 After fractures and surgical anesthesia, hypoxia often worsens, which can cause damage to myocardial cells. Meanwhile, research has shown that for patients with COPD, cardiovascular disease is a common comorbidity and cause of death. Compared with patients without COPD, patients with COPD have a higher incidence of heart failure and other cardiovascular complications.19 Therefore, for patients with COPD, preventive measures such as preoperative oxygen therapy and nebulization should be taken.
ASA grading is a system for quantifying the risks of anesthesia and surgery. Generally, ASA grading can be divided into 6 levels, with 3 levels referring to patients with severe systemic diseases, certain functional impairments, and barely able to tolerate surgical anesthesia. Our results found that ASA ≥3 is the risk factor for postoperative AHF. A cohort research of 170,193 patients in Sweden found that for hip fracture patients with an ASA score of 4, they are more susceptibility to pneumonia, heart failure, and a second hip fracture,20 which was similar to our results. The mechanism behind this can be explained as patients with higher ASA scores have a greater burden of comorbidities, surgery and postoperative immobilization have led to a state of catabolism and have a negative impact on previously existing chronic diseases.21
The current research on the correlation between preoperative waiting time and postoperative related adverse events is controversial. The waiting time from admission to surgery has been studied as a potential modifiable risk factor for complications and death.22–24 A nationwide cohort study of 63,998 patients showed that for patients with ASA scores 3 or 4, they had an increased risk of postoperative complications, such as heart failure, atrial fibrillation, and ischemic events when waiting for more than 24 hours before surgery.25 Some research have also shown that surgery lasting more than 24 hours does not have a significant impact on patient mortality, and implementing strict preoperative time limits for different patients may not be the best strategy.26 Our research results found that waiting time before surgery exceeding 72 hours is a risk factor for postoperative AHF, which may be related to the large number of fracture patients in our hospital, many patients are unable to undergo surgery immediately after the fracture. At the same time, in our hospital, patients often have negative events such as poor blood sugar control, cardiovascular complications, anemia, and thrombosis, these factors can lead to an increase in the average preoperative waiting time of patients. For our results, it can be explained that prolonged preoperative waiting time leads to inflammation, hypercoagulability, catabolism, and stress in patients, at the same time, restricting exercise and fasting after fractures ultimately increase the risk of complications.27 Shortening preoperative waiting time can reduce patients’ exposure to harmful situations, potentially reducing the risk of related complications and mortality.21
Our research results also showed that age is a risk factor for AHF, and the older the age, the higher the probability of AHF. Previous studies have mentioned heart failure syndrome, caused by the aging process, which leads to a series of physiological and biological changes in our heart.28 The mechanisms can be summarized as oxidative stress, inflammatory response, and limited myocardial regeneration ability.29 As age increases, the renewal ability of myocardial cells decreases, in the first 20 years of life, myocardial cells have the highest renewal ability, while the rate of myocardial cell renewal in older adults is significantly reduced,30 leading to an increase in myocardial cell vulnerability.
In our research, general anesthesia accounted for 67.2%, and our research results indicate that anesthesia method is not a risk factor for postoperative heart failure. Anesthesia is mainly divided into regional anesthesia and general anesthesia. The results of a meta-analysis indicate that the anesthesia method for hip fracture surgery is not associated with postoperative complications such as AHF, myocardial infarction, acute renal failure, pneumonia, and cerebrovascular disease, which seems to be similar to our research findings.31 The type of anesthesia is a modifiable risk factor for postoperative outcomes during hip fracture surgery. Compared to general anesthesia, local anesthesia may be more easily accepted by patients. And recent research have also shown that nerve axis anesthesia can reduce the incidence of postoperative complications compared to general anesthesia,32 But further large-scale RCT are needed to support the impact of anesthesia types on postoperative outcomes.
Our results indicate that preoperative atrial fibrillation is not a risk factor for postoperative heart failure. In our research, the incidence of atrial fibrillation is 12.3%, higher than Fu et al 2023 research 7.1% due to combined with CHD in our patients.33 Although some studies have shown that a large proportion of patients develop heart failure after atrial fibrillation34 and older patients have a higher risk of heart failure than stroke after atrial fibrillation.35 But in our research, the occurrence of atrial fibrillation in our research is acute atrial fibrillation caused by post-fracture stress and ischemia. It usually lasts for a short period of time and has a relatively small impact on the patients heart function. We also administer anti-arrhythmic drugs timely. Therefore, it is possible that this brief arrhythmia may not have a significant impact on the patient’s postoperative heart failure.
The shortcomings of our article are: firstly, this study is a retrospective design, which may have some selective bias. Secondly, although the internal validation shows great discrimination. The calibration curve shows that the predicted result and the actual results have good consistency, additional databases need to be used for external validation. Thirdly, due to the limitations of retrospective research, some data such as regional anesthesia types, intraoperative hypothermia, and intraoperative hypotension were not recorded in detail in our case system. Therefore, in the future, we will carry out forward-looking research to collect more comprehensive data, and increase the reliability of conclusions.
In conclusion: for older patients with hip fractures combined with CHD, the prevalence of postoperative AHF was 42.8%, anesthesia type, previous CHD treatment and preoperative atrial fibrillation do not affect postoperative AHF. Fracture type, anemia at admission, ASA ≥ 3, surgical delay time >72 hours, and combined with COPD are risk factors for postoperative acute heart failure. We have developed a nomogram prediction model that can provide an effective personalized guidance plan for clinical practice.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the institutional review board of the third Hospital of Hebei Medical University in compliance with the Helsinki and an exemption from the informed consent was obtained. All data were anonymized before the analysis to safeguard patient privacy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yuan J, Zhu G, Zhao Y, et al. Effect of Hip fracture on prognosis of acute cerebral infarction. Clinics. 2021;76:e3059. doi:10.6061/clinics/2021/e3059
2. Cha YH, Ha Y-C, Ryu H-J, et al. Effect of heart failure on postoperative short and long-term mortality in elderly patients with Hip fracture. Injury. 2020;51(3):694–698. doi:10.1016/j.injury.2020.01.004
3. Tamamura Y, Matsuura M, Shiba S, et al. Heart failure assessed based on plasma B-type natriuretic peptide (BNP) levels negatively impacts activity of daily living in patients with Hip fracture. PLoS One. 2020;15(8):e0237387. doi:10.1371/journal.pone.0237387
4. Tamamura Y, Matsuura M, Shiba S, et al. Effect of heart failure and malnutrition, alone and in combination, on rehabilitation effectiveness in patients with Hip fracture. Clin Nutr ESPEN. 2021;44:356–366. doi:10.1016/j.clnesp.2021.05.014
5. You F, Ma C, Sun F, et al. The risk factors of heart failure in elderly patients with Hip fracture: what should we care. BMC Musculoskelet Disord. 2021;22(1):832. doi:10.1186/s12891-021-04686-8
6. Tian M, Li W, Wang Y, et al. Risk factors for perioperative acute heart failure in older Hip fracture patients and establishment of a nomogram predictive model. J Orthop Surg Res. 2023;18(1):347. doi:10.1186/s13018-023-03825-2
7. Luo Y, Jiang Y, Xu H, et al. Risk of post-operative cardiovascular event in elderly patients with pre-existing cardiovascular disease who are undergoing Hip fracture surgery. Int Orthop. 2021;45(12):3045–3053. doi:10.1007/s00264-021-05227-7
8. Liu ZJ, Yu C-H, Xu L, et al. Risk factors for perioperative major cardiac events in Chinese elderly patients with coronary heart disease undergoing noncardiac surgery. Chin Med J. 2013;126(18):3464–3469. doi:10.3760/cma.j.issn.0366-6999.20122793
9. Xu L, Yu C, Jiang J, et al. Major adverse cardiac events in elderly patients with coronary artery disease undergoing noncardiac surgery: a multicenter prospective study in China. Arch Gerontol Geriatr. 2015;61(3):503–509. doi:10.1016/j.archger.2015.07.006
10. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
11. Carpintero P. Complications of Hip fractures: a review. World J Orthop. 2014;5(4):402–411. doi:10.5312/wjo.v5.i4.402
12. Roshanov PS, Sheth T, Duceppe E, et al. Relationship between perioperative hypotension and perioperative cardiovascular events in patients with coronary artery disease undergoing major noncardiac surgery. Anesthesiology. 2019;130(5):756–766. doi:10.1097/ALN.0000000000002654
13. Sim YE, Sim S-ED, Seng C, et al. Preoperative anemia, functional outcomes, and quality of life after hip fracture surgery. J Am Geriatr Soc. 2018;66(8):1524–1531. doi:10.1111/jgs.15428
14. Min L, Linyi Y, Chen L, et al. Preoperative moderate to severe anemia is associated with increased postoperative major adverse cardiac and cerebral events and pulmonary complications: a propensity score-matched analysis in Hip fracture surgery patients over 80 years old. Perioper Med. 2023;12(1):56. doi:10.1186/s13741-023-00349-5
15. Bolliger D, Mauermann E, Buser A. Preoperative anaemia in cardiac surgery: preoperative assessment, treatment and outcome. Br J Anaesth. 2022;128(4):599–602. doi:10.1016/j.bja.2021.12.049
16. Hazen Y, Noordzij PG, Gerritse BM, et al. Preoperative anaemia and outcome after elective cardiac surgery: a Dutch national registry analysis. Br J Anaesth. 2022;128(4):636–643. doi:10.1016/j.bja.2021.12.016
17. Tian S, Li H, Liu M, et al. Dynamic analysis of perioperative hidden blood loss in intertrochanteric fractures. Clin Appl Thromb Hemost. 2019;25:1076029618823279. doi:10.1177/1076029618823279
18. Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi:10.1056/NEJMoa0808836
19. de Luise C, Brimacombe M, Pedersen L, et al. Chronic obstructive pulmonary disease and mortality following Hip fracture: a population-based cohort study. Eur J Epidemiol. 2008;23(2):115–122. doi:10.1007/s10654-007-9211-5
20. Meyer AC, Eklund H, Hedström M, et al. The ASA score predicts infections, cardiovascular complications, and hospital readmissions after Hip fracture – A nationwide cohort study. Osteoporos Int. 2021;32(11):2185–2192. doi:10.1007/s00198-021-05956-w
21. Hedström M, Ljungqvist O, Cederholm T. Metabolism and catabolism in Hip fracture patients: nutritional and anabolic intervention–A review. Acta Orthop. 2006;77(5):741–747. doi:10.1080/17453670610012926
22. Alvi HM, Thompson RM, Krishnan V, et al. Time-to-surgery for definitive fixation of hip fractures: a look at outcomes based upon delay. Am J Orthop. 2018;47(9). doi:10.12788/ajo.2018.0071
23. Ryan DJ, Yoshihara H, Yoneoka D, et al. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma. 2015;29(8):343–348. doi:10.1097/BOT.0000000000000313
24. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994–2003. doi:10.1001/jama.2017.17606
25. Greve K, Ek S, Bartha E, et al. Waiting more than 24 hours for Hip fracture surgery is associated with increased risk of adverse outcomes for sicker patients: a nationwide cohort study of 63,998 patients using the Swedish Hip Fracture Register. Acta Orthop. 2023;94:87–96. doi:10.2340/17453674.2023.9595
26. Greve K, Modig K, Talbäck M, et al. No association between waiting time to surgery and mortality for healthier patients with Hip fracture: a nationwide Swedish cohort of 59,675 patients. Acta Orthop. 2020;91(4):396–400. doi:10.1080/17453674.2020.1754645
27. Borges FK, Bhandari M, Guerra-Farfan E. Accelerated surgery versus standard care in Hip fracture (Hip ATTACK): an international, randomised, controlled trial. Lancet. 2020;395(10225):698–708. doi:10.1016/S0140-6736(20)30058-1
28. Jugdutt BI. Aging and heart failure: changing demographics and implications for therapy in the elderly. Heart Fail Rev. 2010;15(5):401–405. doi:10.1007/s10741-010-9164-8
29. Dutta D, Calvani R, Bernabei R, et al. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res. 2012;110(8):1125–1138. doi:10.1161/CIRCRESAHA.111.246108
30. Lázár E, Sadek HA, Bergmann O. Cardiomyocyte renewal in the human heart: insights from the fall-out. Eur Heart J. 2017;38(30):2333–2342. doi:10.1093/eurheartj/ehx343
31. Chen DX. Perioperative outcomes in geriatric patients undergoing Hip fracture surgery with different anesthesia techniques: a systematic review and meta-analysis. Medicine. 2019;98(49):e18220.
32. Wang MT. General versus neuraxial anesthesia on clinical outcomes in patients receiving hip fracture surgery: an analysis of the ACS NSQIP database. J Clin Med. 2023;12:11.
33. Fu M, Zhang Y, Zhao Y, et al. Characteristics of preoperative atrial fibrillation in geriatric patients with Hip fracture and construction of a clinical prediction model: a retrospective cohort study. BMC Geriatr. 2023;23(1):310. doi:10.1186/s12877-023-03936-9
34. Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–492. doi:10.1161/CIRCULATIONAHA.115.018614
35. Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–364. doi:10.1016/S0002-9343(02)01236-6
36. Söderqvist A, Ekström W, Ponzer S, et al. Prediction of mortality in elderly patients with Hip fractures: a two-year prospective study of 1944 patients. Gerontology. 2009;55(5):496–504. doi:10.1159/000230587
[ad_2]
Source link






