[ad_1]
research design
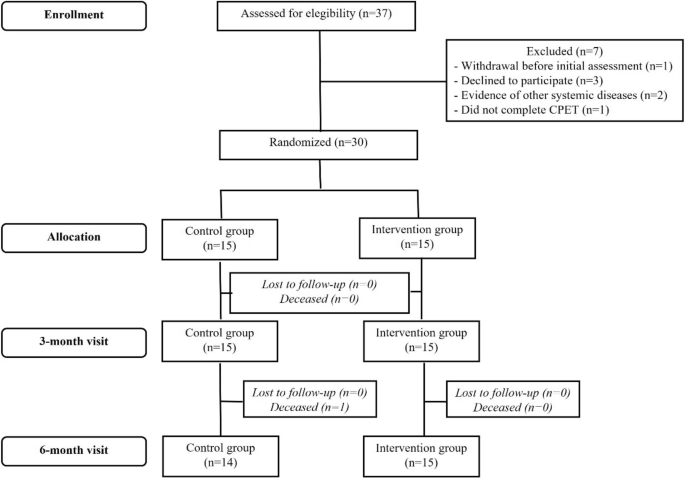
A complete description of the PEACH study and the main results of clinical and functional variables (“Chagas Heart Disease Exercise Program” in Portuguese) has been previously published.19,20. Briefly, the PEACH study was a single-center superiority randomized study of exercise training versus no exercise training (control) conducted at the Evandro Chagas National Institute of Infectious Diseases (INI) from March 2015 to January 2017. It was a parallel group clinical trial. Oswaldo Cruz Foundation (Fiocruz). Those who were followed up at INI were sequentially recruited to participate in the study. Samples included gender, age 18 years or older, CCC, and left ventricular ejection fraction (LVEF). < 45%、HF 症状 (CCC) の有無にかかわらず診断された CD 患者 (2 つの異なる血清学的検査で確認) で構成されました。 、それぞれステージB2またはC)、研究登録前の3か月間にニューヨーク心臓協会(NYHA)の機能クラスIまたはII、臨床的に安定しており、研究登録前の6週間にわたってHFガイドラインに従って最適な薬物療法を受けている。 除外基準は、運動トレーニングを妨げる可能性のある主要な併存疾患または制限の存在、妊娠、週 3 回の運動セッションに参加できないこと、ベースライン (> Regular exercise training was carried out (1 week) for 3 months prior. research, smoking, or related non-CCC evidence.
Eligible patients were randomly assigned to two groups in a 1:1 ratio using sealed envelopes filled with computer-generated sequences using WinPepi software. Sequences were generated in blocks and stratified according to her CCC stage (B2 and C) by her single researcher not involved in recruitment.
In calculating the sample size for this study, a significant mean difference between groups on SF-36 role limitations due to physical problems was considered to be 42.98 points, with a standard deviation of 39.43 points in the control group and 34.32 points in the intervention group. Ta.twenty one. Assuming α = 0.05 and β = 0.20, 26 participants were required (13 in the intervention group and 13 in the control group).
measurement
Patients’ sociodemographic (age, gender, schooling, and self-reported race), anthropometric, clinical, cardiac function, and maximal progressive cardiopulmonary exercise testing (CPET) variables are being evaluated initially. Retrieved from
Schooling includes years of formal study and is stratified into two categories: less than 9 years and more than 9 years. Self-reported race was reclassified into white and non-white (including black, mulatto, indigenous, and yellow people). Anthropometric assessment consisted of height and weight measurements, according to Lohman et al.twenty two. Body mass index (BMI) was calculated as the ratio of weight (kg) to height squared (m).2) and classified according to the World Health Organization definitions.twenty three. CCC stage, NYHA functional class, presence of ECG abnormalities (ventricular extrasystoles, sustained and nonsustained ventricular tachycardia, right branch block, left anterior hemiblock, nonspecific ventricular repolarization changes, atrial fibrillation, clinical variables such as primary ventricular ventricular block). (first-degree atrioventricular block, and second-degree atrioventricular block), and medications were obtained from medical records. Systolic cardiac function was determined by left ventricular ejection fraction (LVEF) using modified Simpson’s rule.twenty four. His CPET with maximal symptom limitation was performed on a treadmill (Imbramedo, Porto Alegre, Brazil) with ramp protocol and active recovery using VO.the year of 2000 Gas analyzer (MedGraphics, St. Paul, MN, USA) connected to a computerized Ergo PC Elite system (Micromed, DF, Brasaria, Brazil).
QoL was assessed using the Portuguese version of the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) questionnaire at baseline, 3 months later, and at the end of follow-up (6 months) .25, 26, 27. The instrument has high internal consistency, test-retest reliability, and can build validity in Brazilian populations across different age groups and health conditions, including patients with CCC.11,27,28,29,30,31,32. The SF-36 consists of 36 questions covering the 4 weeks prior to the interview and addresses eight different measures: physical functioning, role limitations due to physical problems, body pain, general health perception, vitality, and social functioning. A general multidimensional tool consisting of 36 separate questions. , role limitations due to emotional problems or mental health. These scales define his two summary scores: Physical Component Summary (PCS) and Mental Component Summary (MCS). Both PCS and MCS have contributions from all eight scales, but the first four scales of PCS and the last four scales of MCS have higher weights. The final score ranges from 0 (worsening QoL) to 100 (best QoL).25,26.
intervention
Patients randomized to the exercise group performed physical exercise sessions for 60 minutes, three times a week for 6 months. Each session consists of 30 minutes of aerobic exercise on a treadmill or cycle ergometer, 20 minutes of strength training consisting of 2 sets of 12 repetitions of major muscle groups (sit-ups, push-ups, pull-ups), and 10 minutes of strength training It has been. About stretching exercises. The intensity of aerobic exercise was set according to the anaerobic threshold heart rate obtained during CPET (90–100% of the anaerobic threshold heart rate during the first month of exercise training, thereafter is 100-110% of the anaerobic threshold heart rate)33. Anaerobic thresholds used to determine target heart rate are based on baseline cardiopulmonary exercise tests for training sessions conducted at baseline and month 3, and for training sessions conducted at month 3 and month 6. Derived from 3 months of cardiopulmonary exercise testing. For patients in whom no anaerobic threshold was identified during CPET (n = 13; 43%), training intensity was prescribed according to the Hellerstein formula [HRâ=â(102â+âmaximum metabolic equivalents achieved)/1.41)]34In this case, the target heart rate range is from 70% of the maximum heart rate obtained with CPET to the Hellerstein percentage for the first month, and from the Hellerstein percentage to 85% of the maximum heart rate for the first month. It was up to %. The next few months. The Borg scale was used as an adjuvant to the exercise intensity prescription (targeting 2 to 4 on the CR10 Borg scale). Exercise intensity was controlled by an exercise physiologist who supervised all exercise sessions. All exercise sessions were center-based and conducted in the morning in a temperature-controlled indoor environment under multidisciplinary supervision (including an exercise physiologist and a physician). Participants were not given any instruction on home exercise (other than the center-based program). Patients in the control group were not provided with a formal exercise prescription.
During the study, patients in both groups had monthly consultations with a cardiologist based on the recommendations of the Brazilian Consensus on CD.35. Additionally, both groups received identical nutritional and pharmaceutical counseling during the study. Nutritional counseling consists of general instruction on healthy eating habits, such as reducing intake of saturated fatty acids and increasing intake of polyunsaturated and monounsaturated fatty acids, vitamins, and high-fiber carbohydrates. I did. Reductions in sodium consumption and water intake were also promoted in HF patients. Pharmaceutical care consists of instruction on drug use, drug dosage, and compliance, as well as the monthly distribution of individual packages containing tablets organized by time and day to be taken according to the prescription. Ta.19.
data analysis
Descriptive analyzes consisted of means and standard deviations for continuous variables and number of observations and percentages for categorical variables. A longitudinal analysis of the impact of exercise-based CR on QoL was performed using a mixed linear model. This approach allows us to evaluate the interaction term (group x time) to estimate the rate of change in the outcome between groups. The model was fitted by adjusting the respective baseline QoL values. All participants, regardless of adherence or loss to follow-up, were included in statistical analyzes to characterize intention-to-treat analyses. A line graph was created to visually illustrate the rough trajectory of her QoL scale during follow-up for each group.
The Research Electronic Data Capture (REDCap) web application was used for data management, and data analysis was performed using Stata 13.0. Statistical significance was set as follows. p±0.05 for all analyses.
ethical considerations
All participants received information about the study goals and procedures and voluntarily agreed to participate by signing a written informed consent. This study was conducted in accordance with Resolution 466/2012 of the Brazilian National Health Council and was approved by the Institutional Research Ethics Committee (CAAE: 38038914.6.0000.5262) in February 2015. The clinical trial was registered at ClinicalTrials.gov (NCT02517632).
[ad_2]
Source link






