[ad_1]
April 6, 2024 — A study shows that patients with heart failure who had a small shunt inserted between the left and right atria of the heart experienced significantly lower rates of pain compared to those who received a placebo treatment after a median follow-up of 22 months. No significant overall effect was found in the study presented at the annual scientific session of the American College of Cardiology.
The trial, called RELIEVE-HF, is the first randomized placebo treatment of interatrial shunts in patients with both major types of heart failure: heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). This is a controlled test. . Although the trial did not meet its primary endpoint, it advances the field by providing a signal that the benefits and risks of interatrial shunts may vary by type of heart failure, researchers said. That’s what it means.

Greg Stone, MD
“When we looked at outcomes in patients with heart failure across a wide range of left ventricular ejection fractions, the Ventura interatrial shunt was very safe but did not improve outcomes compared to no treatment. In the specified analysis, data suggest that shunts may be beneficial for patients with HFrEF but may worsen outcomes for patients with HFpEF,” said the Department of Cardiology at the Icahn School of Medicine at Mount Sinai. said Greg Stone, MD, professor of population health sciences. in New York State and lead author of the study. “We believe further studies are needed to confirm the benefits observed in patients with reduced ejection fraction.”
Heart failure is a condition in which the heart becomes too weak or stiff to pump blood effectively, causing fatigue, organ damage, shortness of breath, and an increased risk of life-threatening cardiovascular events. In HFrEF, the heart muscle becomes weak and cannot tighten as tightly as it should. In HFpEF, the left ventricle becomes stiff and does not fill properly with blood.
The Ventura shunt is one of several interatrial devices being tested to help treat heart failure. Forms a small connection or passageway between the left atrium and the right atrium, specifically designed to allow blood to flow out of the left atrium when left atrial pressure increases, thereby reducing pressure in the left atrium and lungs. Masu. Elevated left atrial pressure is a major cause of breathlessness and hospitalization associated with heart failure.
The trial randomly assigned 508 patients at 94 sites in North America, Europe, Israel, Australia, and New Zealand. All participants had symptomatic heart failure despite taking the drug at the maximum tolerated dose. Approximately 40% of participants had HFrEF and 60% had HFpEF.
Participants were randomly assigned to undergo a procedure to insert a Ventura shunt or a placebo procedure, all following a script with the same protocol to mask whether a shunt was inserted or not. The operators knew which procedure each patient received, but the patients, their families, and the rest of the medical team caring for the patients after the procedure did not. The researchers tracked each participant’s outcomes for at least one year and up to two years.
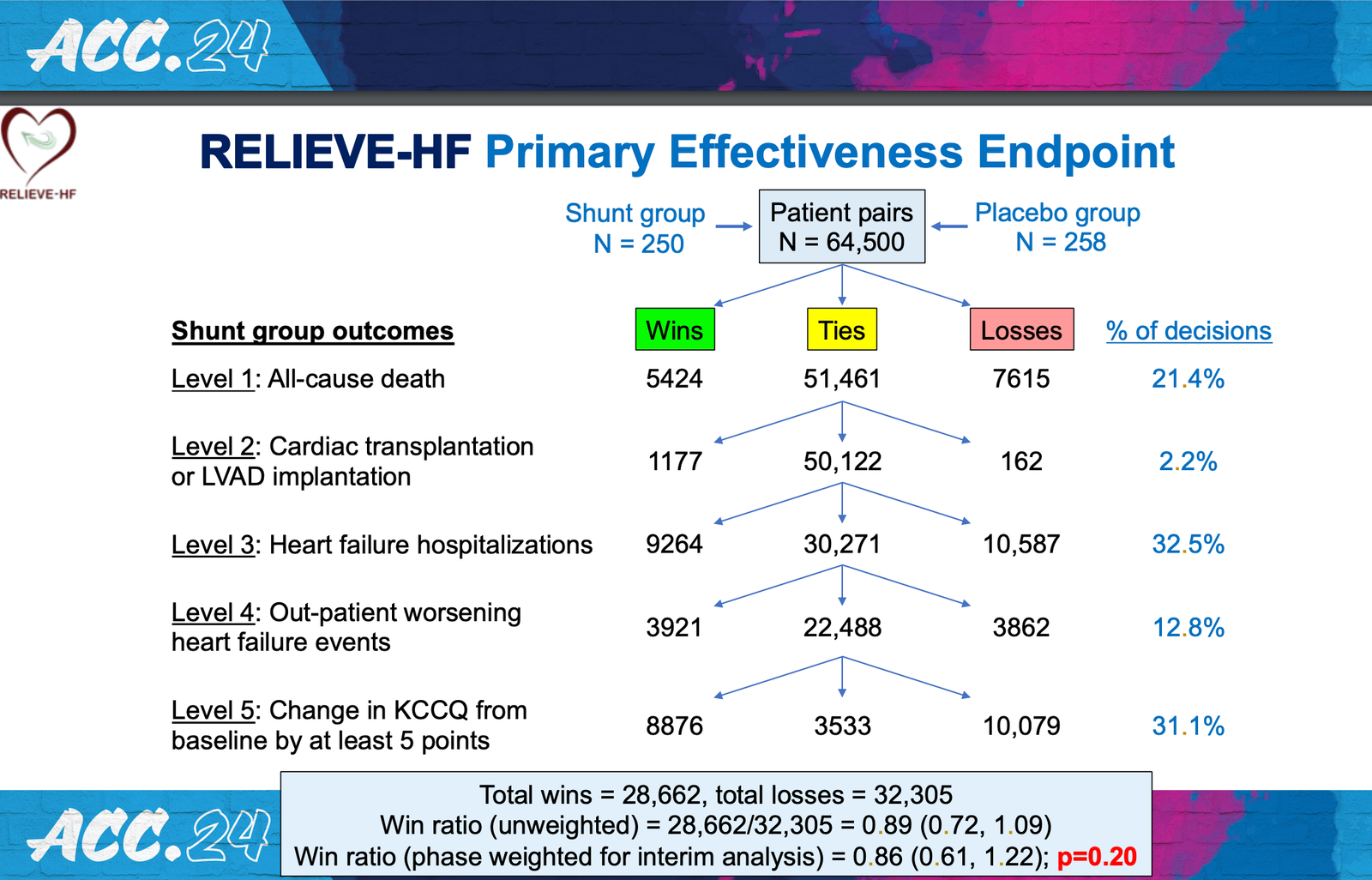
There were no significant differences between the groups in the study’s primary endpoint, a hierarchical composite ranking of death from any cause. Heart transplant or left ventricular assist device. Hospitalization due to heart failure. Worsening outpatient heart failure event. Changes in quality of life measured using the Kansas City Cardiomyopathy Questionnaire (KCCQ). This hierarchical composite approach to assessing efficacy allows different types of outcomes to be incorporated in a ranked format into an overall “win rate” that reflects the overall outcome of a drug or device. .
In a pre-planned analysis focused on heart failure type, HFrEF patients who received a shunt had improvements across all outcomes assessed (particularly fewer hospitalizations for heart failure), whereas HFpEF who received a shunt It turns out that the number of patients is increasing. Mortality and heart failure hospitalization rates. This difference may be due to HFrEF’s greater myocardial compliance and flexibility, which may more easily accommodate the extra blood flowing into the right atrium, Stone said.
There were no significant device-related or procedure-related cardiovascular or neurological adverse events in either group during the study period.
Remarkably, significant improvements in quality of life as measured by the KCCQ were observed in all groups, including those receiving placebo treatment in both HFrEF and HFpEF, demonstrating that this measure is a reliable measure of quality of life outcomes. This suggests that it may not be an indicator. Stone said in this context.
“There was an incredible placebo effect,” he says. “These observations, particularly the fact that quality of life improved in HFpEF patients, who were more likely to be hospitalized for heart failure and had lower survival rates after shunt treatment, suggest that this quality of life measure in this type of disease It raises questions about the interpretation of the “ordeal.” ”
Differences in outcomes observed between people with different types of heart failure may inform future research and development of interatrial devices, but the researchers believe that this trial does not reflect the differences between the two types of heart failure. He said that he does not have the ability to demonstrate Therefore, these results should be considered exploratory. They also stated that the results may not apply to other interatrial shunts other than the Ventura shunt.
This study was funded by V-Wave Medical.
Dr. Stone will present the study, “A Double-Blind, Randomized, Placebo Procedure Controlled Trial of Interatrial Shunts in Patients with HfrEF and HfpEF: Key Results of the RELIEVE-HF Trial” on Saturday, April 6, 2024, at 9 p.m. . 30:00 a.m. ET/1:30 p.m. UTC in the main tent in Hall B-1.
For more information, please visit www.acc.org.
Click here for detailed coverage of the ACC24 conference.
[ad_2]
Source link






