
[ad_1]
Study population
This was a single-center study, retrospectively reviewing hospitalized HFpEF patients who underwent DECT examination at the Second Affiliated Hospital of Nantong University. From January 2019 to December 2020, 125 patients were enrolled in the study. HFpEF, defined according to the 2016 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure16. Inclusion criteria were: (1) presence of signs or symptoms of heart failure and left ventricular ejection fraction (LVEF) ≥50%; (2) B-type natriuretic peptide (BNP) ≥100 pg/ml or N-terminal pro-BNP (NT-pro BNP) ≥300 pg/ml. (3) New York Heart Association (NYHA) grade II or higher. (4) the patient had hematocrit and her NT-pro-BNP measurements performed within her 24-hour interval of her DECT; Unique criteria include: (1) Contraindications to iodinated contrast media. (2) Patients with estimated glomerular filtration rate (eGFR) ≦ 30 ml/min/1.73 m2. (3) have a history of acute coronary syndrome or myocardial infarction, or severe valvular heart disease (i.e., moderate or greater left-sided valvular disease); Known or suspected hypertrophic/infiltrative cardiomyopathy, constrictive pericarditis, amyloidosis, adult congenital heart disease. (4) Previous percutaneous coronary intervention or coronary artery bypass grafting.
Ethics approval
This study complied with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Second Affiliated Hospital of Nantong University (No. 2020KN094). All patients signed an informed consent form.
Baseline characteristics and DECT scan protocol
We collected baseline characteristics, including age, gender, body mass index (BMI), smoking, alcohol consumption, hypertension, diabetes, atrial fibrillation, and blood pressure, and clinical data from hospital medical records. Fasting venous blood was collected and biochemical tests were performed on the second morning after the patient was admitted. Hemoglobin, fasting plasma glucose (FPG), glycated hemoglobin A1c (HbA1c), serum creatinine (Scr), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and NT-pro BNP levels were detected.
All patients were examined with third-generation dual-source CT (Somatom Force, Siemens Healthcare, Forchheim, Germany). Before the test, if the patient’s heart rate exceeds her 75 bpm, 25-50 mg of metoprolol (AstraZeneca Pharmaceuticals Co., Ltd.) will be administered to lower the heart rate. Next, a bolus of 50 ml of iopromide (Ultravist 370, Bayer Pharmaceuticals & Healthcare Co., Ltd.) was injected into the antecubital vein at a flow rate of 4.5–5.0 ml/s, followed by 30 ml of normal saline. . Bolus chasers are injected at the same flow rate.
The CCT scan included the following procedures: prospective electrocardiogram (ECG)-gated calcium score acquisition, prospective ECG-gated coronary CT angiography (CCTA), and delayed DECT scan. The scan ranged from 1 cm below the carina to the diaphragm level of the heart. Scan parameters included A tube voltage 100 kV, B tube SN140 kV, automatic tube current modulation technique, 192 × 0.6 mm collimation, and 0.15 pitch factor. The acquisition phase was 65–80% of the RR interval. DECT scans were performed 7 minutes later using the same parameters and scan range for calcification score acquisition. All images were reconstructed with a matrix of 512 × 512, slice thickness of 0.6 mm, spacing of 0.4 mm, and convolution kernel (Qr36). The effective radiation dose for CT was calculated by multiplying the dose-length product by a conversion factor of 0.014.
DECT data post-processing and ECV measurement
DECT post-processing was performed on a workstation (Syngo via, VB20, Siemens Medical Solutions, Forchheim, Germany). The iodine map was constructed by the “Heart PBV” software based on the material decomposition method to display the distribution of iodine within the left ventricle. The iodine map was reconstructed in an 8-mm-thick short-axis image without gaps from the base of the heart to the apex.According to the 16 segments of the left ventricular myocardium17, a region of interest (ROI) was manually drawn sparingly in each segment to avoid surrounding the myocardium. ROI in blood pools with size greater than 100mm2 Papillary muscles and focal delayed myocardial enhancement were excluded. ECV evaluation was completed by her two experienced observers. DECT-ECV was calculated as follows: ECV (%) = (myocardial iodine concentration/blood pool iodine concentration) × (hematocrit level of 1) × —≧100%, myocardial iodine concentration is delayed enhancement The myocardial iodine concentration is the blood pool iodine concentration, and the blood pool iodine concentration is the enhanced iodine concentration of the left ventricular blood pool (Figures 1, 2).
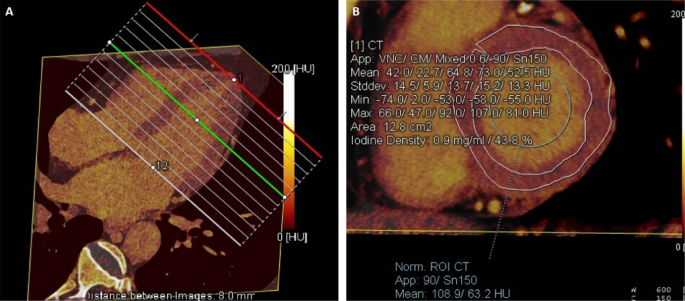
ECV measurements are displayed as low values on the iodine map. (a) The iodine map was reconstructed in a short-axis view with a thickness of 8 mm from base to apex. (B) A 75-year-old man with HFpEF, myocardial iodine/blood iodine was 43.8% in the left ventricular base, 42.5% in the mid-left ventricle, and 36.5% in the ape left ventricle. Serum hematocrit value is 44.2%. The mean ECV of the left ventricular base is calculated to be 24.44%, and the mean ECV of the left ventricular midsection, the apex, and the entire left ventricle is calculated to be 23.72%, 20.2%, and 23.11%.
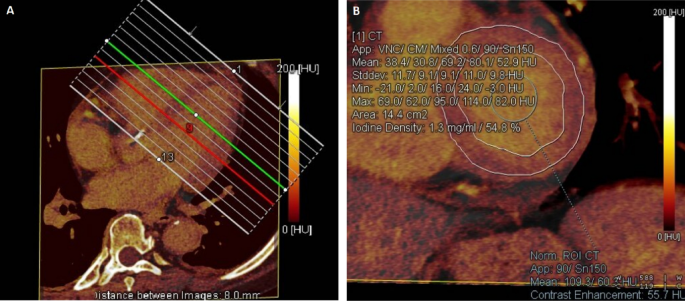
The iodine map shows high ECV measurements. (a) The iodine map was reconstructed in a short-axis view with a thickness of 8 mm from base to apex. (B) A 74-year-old woman with HFpEF, myocardial iodine/blood iodine was 54.8% in the left ventricular base, 51.5% in the mid-left ventricle, and 59.1% in the ape left ventricle. Serum hematocrit value is 32.4%. The mean ECV of the left ventricular base is calculated to be 37.04%, and the mean ECV of the left ventricular midsection, apex, and the entire left ventricle is calculated to be 34.81%, 39.95%, and 36.93%.
Echocardiographic measurements
All study participants underwent transthoracic echocardiography by an experienced echocardiographer using a Philips IE33 ultrasound scanner with an S5-1 transducer. Cardiac structure and function were assessed according to the recommendations of the American Society of Echocardiography and European Society of Echocardiography guidelines.18,19. Briefly, echocardiographic parameters include left ventricular volume, ejection fraction, and diastolic function. Left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), and LVEF were derived from Simpson’s biplane method. Left atrial volume (LAV) was measured using the field length method from apical two-chamber and four-chamber views at ventricular end-systole. This was then divided by the body surface area to obtain the left atrial volume index (LAVI). Peak early diastolic transmitral flow velocity (E wave) and early diastolic mitral annular velocity (eʹ) to estimate LV filling pressure were assessed by Doppler echocardiography. These were then averaged to calculate the E/eʹ ratio.
Follow-up and endpoint confirmation
The study period lasted 12 months. The majority of patients were followed up by reviewing electronic medical records, but telephone interviews were conducted for some participants in special circumstances (such as contacting family members in the event of patient death). The composite endpoint in the current study was defined as hospitalization for HFpEF and all-cause mortality.
statistical analysis
All statistical analyzes were performed by SPSS 22.0 software. Continuous variables were evaluated through the Shapiro-Wilk test and are presented as either the mean and standard deviation (SD) if normally distributed, or the median and interquartile range if non-normally distributed. Categorical variables were presented as numbers and percentages. To compare baseline characteristics of patients with and without the composite outcome, Mann-Whitney U test for non-normally distributed continuous variables, two-tailed t test for normally distributed continuous variables, and χ2 Test for categorical variables. Bland-Altman analysis was performed to assess intraobserver and interobserver agreement for ECV measurements. Correlations between variables and ECV were assessed using Pearson or Spearman correlations. Linear regression analysis was performed to identify factors associated with ECV.
Patients were divided into three groups according to ECV. Kaplan-Meier curves were constructed and the log-rank test was used to measure the differences in outcome events between these groups. Endpoint risk factors were analyzed using both univariate and multivariate adjusted Cox regression analyses. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were presented as well. P±0.05 was recognized as a statistical difference.
[ad_2]
Source link






