[ad_1]

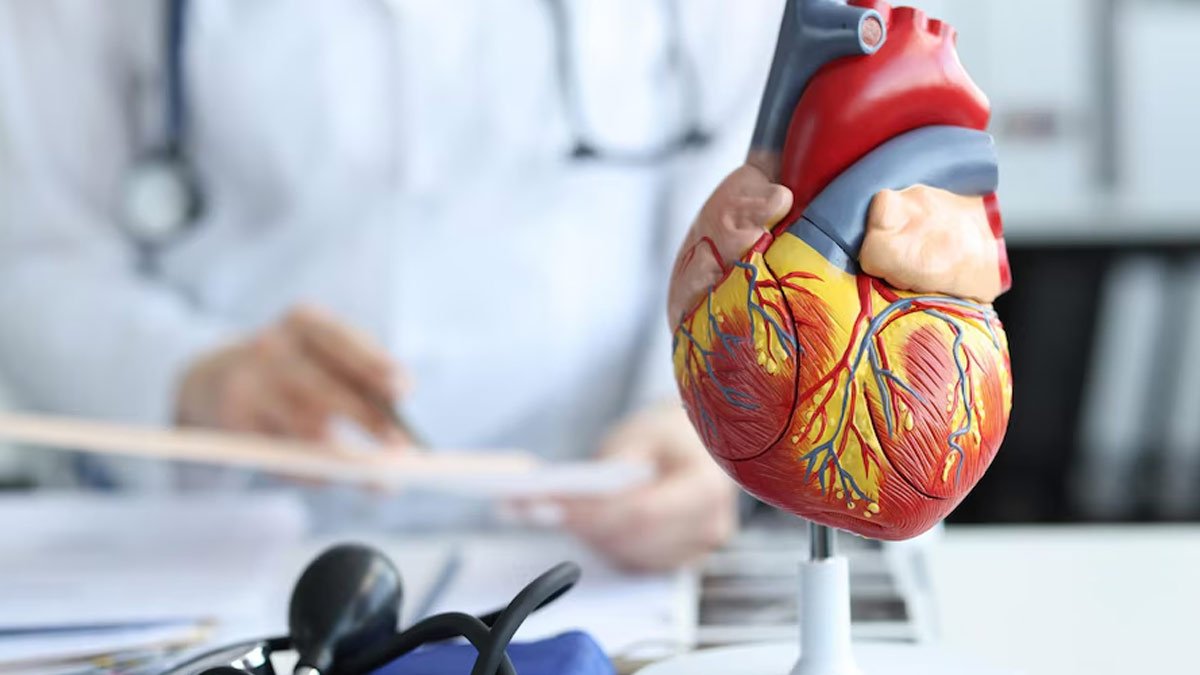
Pulmonary arterial hypertension is a worsening and ultimately life-threatening disease that can lead to worsening lung and heart problems and is treated with drugs to relieve symptoms. The FDA’s approval of Merck’s new drug is the first for a treatment that addresses the root cause of the disease.
Tuesday’s approval of the Merck drug is for the treatment of adults at intermediate or high risk of developing pulmonary arterial hypertension (PAH). The drug, known as sotatercept during development, will be sold under the brand name Winrevair.
Hypertension, or high blood pressure, is common. PAH is a rare form of high blood pressure that specifically affects the artery that carries blood from the right side of the heart to the lungs. When these blood vessels narrow, blood flow to the lungs slows and blood pressure increases. As a result, the heart has to work harder to pump blood to the lungs. This disease can cause heart failure.
Patients with PAH experience shortness of breath, fatigue, and chest pain. Once developed, symptoms gradually worsen and may lead to hospitalization or death. Drugs already used to treat PAH help improve blood flow by relaxing and widening blood vessels. Winrevair is based on research showing that imbalances in cell signaling cause cells to proliferate and thicken the insides of pulmonary blood vessels. PAH patients have elevated levels of a protein called activin, which tips the balance of cell signaling toward cell proliferation. Winrevair is a fusion protein designed to capture activin and other proteins associated with PAH.
The Phase 3 clinical trial evaluated Winrevair in addition to standard-of-care PAH treatment. The drug’s effectiveness was measured with a walk test, a common method for evaluating cardiovascular drugs. The main goal was to administer the drug via subcutaneous injection every three weeks and measure changes in how far patients could walk in six minutes after 24 weeks of treatment.
The primary objective results showed that the median change in walking distance for the study drug group was 34.4 meters, compared to a median change in walking distance of 1.0 meters for the placebo group. The study also achieved statistical significance for eight of nine secondary objectives, including an 84% reduction in all-cause mortality in the Winreair group compared to the placebo group. It also includes things. This improvement was maintained over his 18-24 months of continued treatment with the drug. Phase 3 data was published in the New England Journal of Medicine last year.
“Patients with pulmonary arterial hypertension continue to need new treatment options that support important clinical goals such as increased exercise capacity and improved functional class,” said Brigham and Women’s Hospital Pulmonary said Dr. Aaron Waxman, executive director of the disease center. Researchers on the Merck drug’s Phase 3 trial said in a prepared statement. “Adding sotatercept to background therapy may provide a new standard treatment option for patients with pulmonary arterial hypertension.”
The most common adverse events reported in clinical trials included nasal and gum bleeding, abnormally low platelet levels, and high hemoglobin levels. Winrevair’s label advises clinicians to check hemoglobin and platelet levels before the first five doses and to monitor those levels periodically thereafter. Dose can be adjusted to reduce these complications.
[Paragraph added with analyst comment.] Leerink Partners analyst Dina Graybosch characterized Winreair’s label as a victory for the drug in a note sent to investors Wednesday. The approval broadly covers the treatment of patients with PAH, which is important for reimbursement, she said. There are no black box warnings about toxicity on the label. Bleeding events observed in clinical trials are more likely to occur in patients who are also receiving other treatments, the FDA said. Although the label includes monitoring requirements, Graybosch said this monitoring is focused on patients receiving these combination therapies and those with low platelet counts. Another win for the drug is that its approval includes home administration by patients or caregivers trained in how to administer it.
Winrevair joined Merck in 2021 with its $11.5 billion acquisition of Acceleron Pharma. New PAH drugs are important to Merck. Merck is looking for products to offset future revenue losses as the patent for its top product, cancer immunotherapy Keytruda, expires at the end of 2010. -Products for sale.
Merck said it expects Winrevair to be available by the end of April. The company will supply the PAH drug in single-vial or double-vial kits, priced at $14,000 per vial. Based on experience with the drug in clinical trials, the company expects about two-thirds of patients to use the single-vial kit. So, calculated every three weeks, Winrevair’s annual cost is over $242,000. The Institute for Clinical and Economic Reviews, a nonprofit organization that monitors drug prices, estimated that the cost-effective price for Merck PAH drugs ranges from $17,900 to $35,400 per year. Merck said a patient’s out-of-pocket cost will depend on many factors, including the details of their insurance plan, which may include out-of-pocket limits.
Winrevair is still undergoing regulatory review in Europe. Clinical trials are underway that may expand the use of Winrevair. Phase 3 trials are ongoing in additional groups of patients with PAH. Phase 2 studies are testing the drug in another type of pulmonary hypertension.
Merck & Co. has another PAH drug candidate, MK-5475. Formulated as an inhaler, this small molecule targets enzymes to induce blood vessel relaxation. MK-5475 is currently in Phase 2/3 testing. Other companies offering his PAH drugs in various stages of clinical development include Aerovate Therapeutics, Gossamer Bio, Keros Therapeutics, and Novartis.
[ad_2]
Source link